APCM 2022: Precision Medicine Is Opening Up a New Prospect of Cancer Therapy
Precision medicine has been driving progress in cancer therapy for the last decade. Advanced Precision Cancer Medicine Forum 2022 (APCM), hosted by the Taiwan Oncology Society, Taiwan Clinical Oncology Society, Taiwan Society for Immunotherapy of Cancer, Taiwan Lung Cancer Society, Taiwan Society of Pathology, and ACT Genomics, has gathered over 300 clinicians and experts together. They discussed the latest trends of precision medicine in cancer therapy, including new approaches to diagnose different cancer types and the applications of each cancer test.
Advancing Taiwan’s Precision Medicine: National Health Insurance
In his opening remark, Po-Chang Lee, the Director General of the National Health Insurance Administration (NHIA), highlighted the medical expense of cancer therapy with the coverage of over 154 cancer drugs; the total expenditure has reached over $40.3 billion NT Dollars. To relieve the burden of major disease treatments, the NIHA has established a system to evaluate the efficacy of health insurance. NIHA has combined the patients’ genomic and biomarker data, followed up on the effectiveness of the medicine, and revised the payment system accordingly.
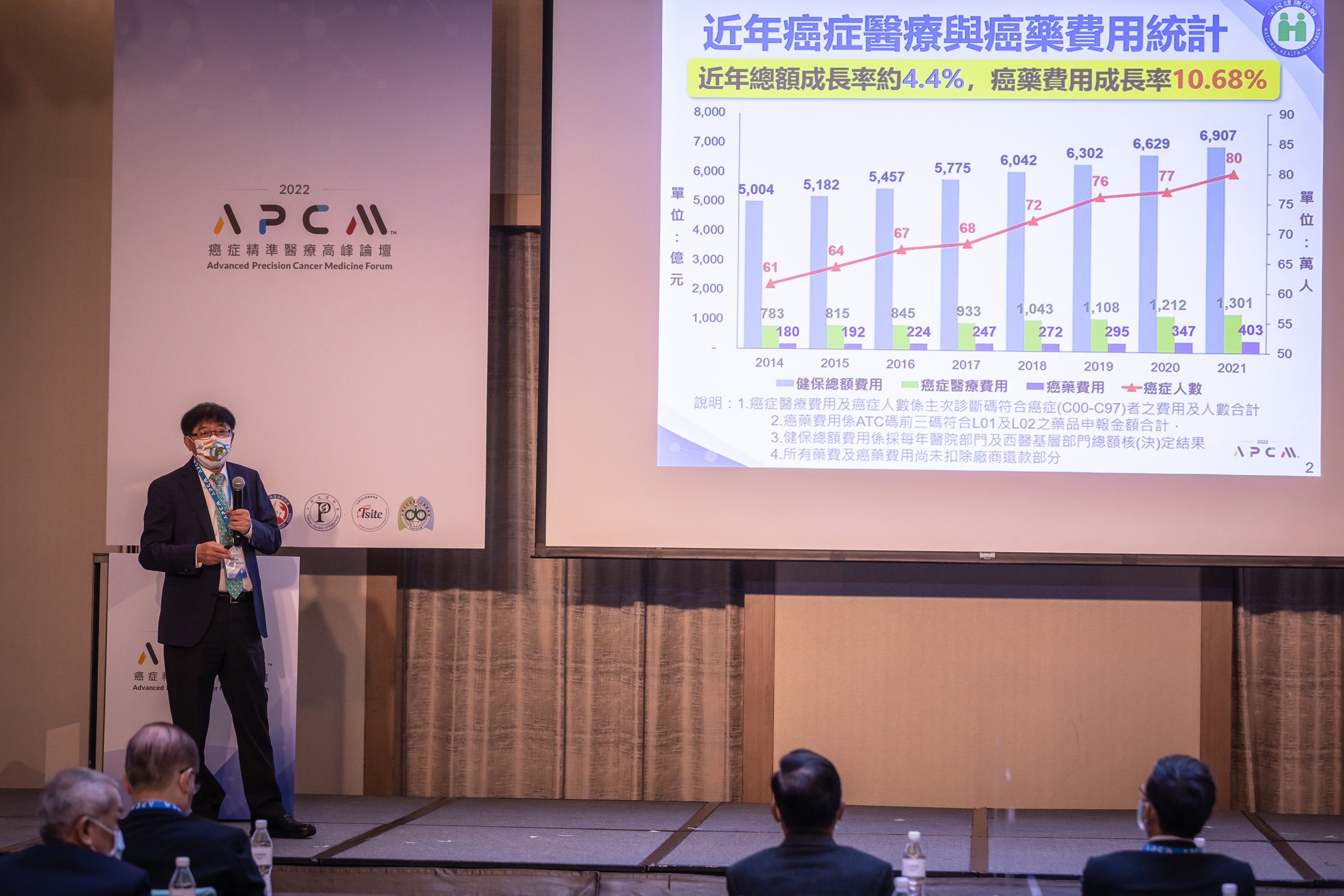
The application of Next-Generation Sequencing (NGS) allows the medical providers to analyze a group of patients’ tumor genes and identify the matching therapies all at once. As a result, it has helped promote precision medicine in treating cancer significantly.
Dr. Lee also pointed out that the health insurance system will improve in three major ways in the near future. Firstly, to accelerate the inclusion of drug testing into the payment coverage. Secondly, to strengthen the medication of precision medicine and adjust the coverage range flexibly based on the Real-World Evidence (RWE). Thirdly, to integrate the clinical data and AI diagnostic system to improve the early efficacy of cancer therapies.
The Era of Target Therapy and the Biobank
Pan-Chyr Yang, the academician of Academia Sinica, reviewed how Taiwan has participated in the progress of precision medicine in the world. Take treating lung cancer as an example; the major breakthrough in target therapy was the world’s first, non-smoker-focused study, Iressa Pan-Asian Study (IPASS), which showed that the EGFR gene mutation is one of the most common biomarkers in Asian patients. Since then, the key factor in the outcome of the lung cancer therapy is the early detection, with over 93% cure rate in zero, first, and second stages of lung cancer.

Dr. Yang further mentioned Taiwan’s National Cancer Control Program has entered stage IV, aiming to establish a genomics and transcriptome database with NGS technology to facilitate the early detection of cancer. It is also known as “stage shift,” meaning to increase the entire outcome of cancer therapy.

Needless to say that the BioBank is the foundation of precision health development. Many countries in East Asia, such as Japan and Korea, have built their national healthcare database to help predict the risk factors for cancer. Starting from 2012, Taiwan BioBank has collected over 200,000 patients’ clinical data, including health, genetic, and environmental factors. By combining the data from Taiwan BioBank and Taiwan Precision Medicine Initiative (TPMI), Taiwanese clinicians are able to predict and analyze cancer risk factors more precisely and efficiently.
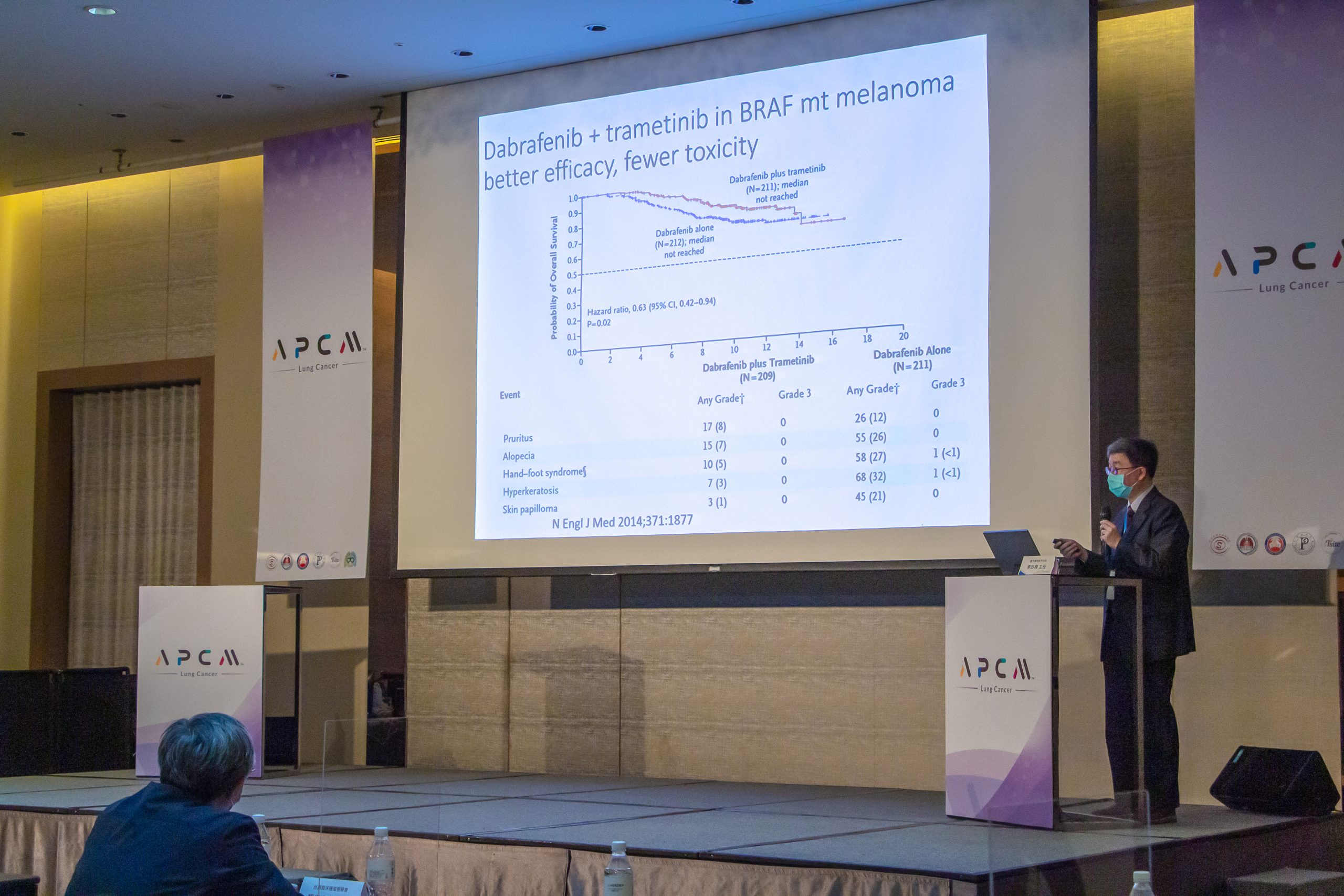
TMB Testing Predicts the Efficacy of Immunotherapy
Dr. Shu-Jen Chen, the Chief Scientific Officer of ACT Genomics, emphasized the importance of biomarker testing, which plays a crucial role in immunotherapy, by introducing the clinical application of tumor mutational burden (TMB). TMB is a critical factor in predicting the outcome and efficacy of immunotherapy. Generally speaking, a high rate of TMB would improve the overall survival of immunotherapy.
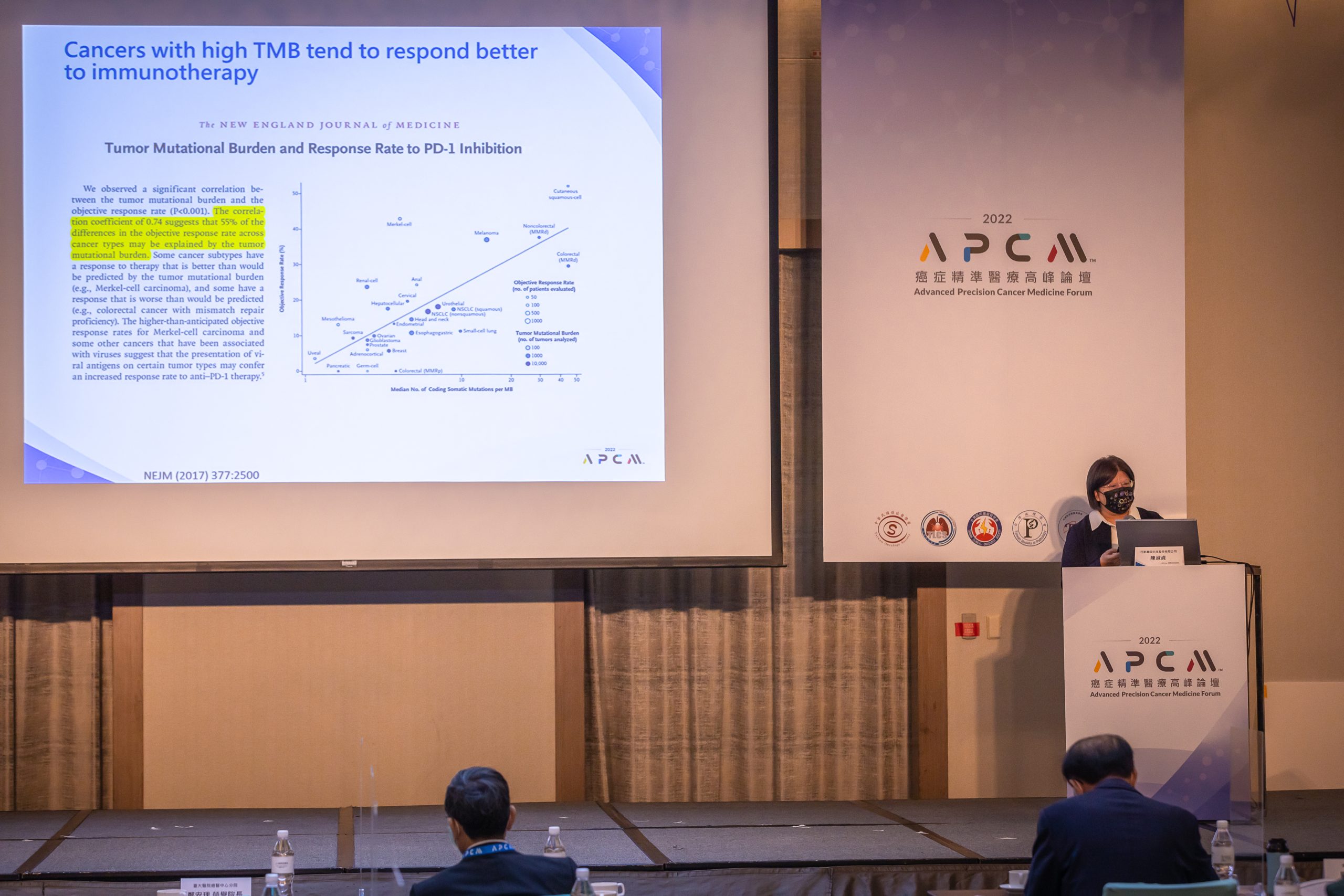
Dr. Chen pointed out that ACT Genomics was the only Asian company participating in the TMB Harmonization Project held by Friends of Cancer Research in 2019. The results of the project have demonstrated the precision and accuracy of the ACT Genomic’s NGS assay on testing performance. Furthermore, it has provided a guideline for establishing the range of TMB cut-off, allowing the clinician to predict the outcome of the immunotherapy accordingly.
In conclusion, TMB is a great biomarker, but it is only accurate in certain conditions. First, the TMB testing should be above 700 kb and combined with a biology information model to evaluate the therapy outcome. Second, the expression of certain genes should also be taken into consideration, such as the drug resistance inducing gene, STK11, or the abnormal expression of MDM2/4, which may cause hyperprogressive growth of the tumor. Last but not least, cross-validation with liquid biopsy or tissue section from samples of different sources is also necessary to confirm the result.
Biomarkers Continue to Improve the Efficacy of Precision Medicine
During the panel discussion, the panelists addressed that air pollution and lifestyle have played a crucial role in carcinogenesis. Thus, it is necessary to combine the data of mutation, methylation, and tumor driver gene to create more precise factors for prediction.

When it comes to the clinical application of tumor biomarkers, accumulation of clinical data and performing multiple testing are both great ways to increase the accuracy of the treatment. As Yang highlighted in the session, the future of cancer therapy will focus on integrating DNA and RNA tests for multiple biomarkers, including known gene mutation, fusion gene, and different types of human leukocyte antigens, providing the clinicians with a more comprehensive picture of the cancer progression in order to perform a better, more personalized cancer therapy.
Written by Kathy Huang
Translated by Aurora Mau









