Clinical trial results of Belief BioMed’s hemophilia B gene therapy drug candidate BBM-H901 published in The Lancet Haematology
SHANGHAI, May 20, 2022 /PRNewswire/ — The early phase clinical trial results of BBM-H901, the first intravenously injectable hemophilia B gene therapy product developed and tested in Asia, are published in The Lancet Haematology, a top international journal in hematology.
https://www.thelancet.com/journals/lanhae/article/PIIS2352-3026(22)00113-2/fulltext
The Institute of Hematology and Blood Diseases Hospital at the Chinese Academy of Medical Sciences conducted the clinical trial in collaboration with Belief BioMed, a leading innovative gene therapy company, and East China University of Science and Technology. The safety and long-term efficacy of this treatment strategy have been demonstrated with significant relief of disease symptoms.
BBM-H901 is molecularly engineered adeno-associated virus (AAV) based gene therapy drug for hemophilia B developed and manufactured by Belief BioMed. The company has the proprietary rights of the AAV capsid and the expression cassette. The novel human liver-tropic AAV capsid can efficiently infect liver cells, greatly shorten the circulation time of drugs in the blood and reduce the immune response generated by the capsid. At the meantime, the novel expression cassette utilizes a mini-liver-specific promoter to drive efficient expression of FIX-padua. Furthermore, the company has achieved several proprietary improvements in process and quality control, forming an advanced, reliable, efficient CMC process, with a single production cell culture volume of up to 500L currently.
“The study was the first clinical trial conducted by Belief BioMed in China. We are very grateful to our partners for their great support throughout the journey,” said Dr. Jane Zheng, CEO of Belief BioMed. “We are very proud to introduce BBM-H901, which is the first intravenously delivered gene therapy drug for hemophilia B in China and the first example of systemic gene therapy for rare diseases in China. We will continue to advance discovery and research of all other pipelines, and to accelerate clinical trials and commercialization of the drugs. We hope gene therapy could be accessible to patients with rare or common diseases as soon as possible.”
The study included 10 patients with moderate to severe hemophilia B (FIX:C<2%) who were intravenously infused with 5×1012vg/kg BBM-H901. A median follow-up of 58 weeks (IQR: 51.5-99.5) was completed, and the FIX:C coagulation factor reached the mean 36.9±20.5IU/dl. Compared with similar international studies, BBM-H901 works rapidly with vector-derived FIX:C expression within 24h following gene therapy. It reached 57.1±20.2IU/dl (mean ± SD) within the first week and peaking at 64.1±22.5IU/dl (mean ± SD) at the median of 5 (1,6) weeks.
Figure 1: Long-Term Expression Level of BBM-H901 Derived FIX:C
Zhang Lei, Professor / Vice Director of Institute of Hematology and Blood Diseases Hospital at the Chinese Academy of Medical Sciences said: “The BBM-H901 study is the first clinical study in China and even Asia to utilize liver-targeted AAV vectors for the treatment of hemophilia B. The safety and long-term effectiveness of this treatment strategy have been well demonstrated with significant relief of associated complications. We believe that this study will certainly provide a reliable foundation and theoretical support for the future clinical application of systemically administered gene therapy drugs, and is also an important guide in promoting the R&D and clinical transformation of gene therapy drugs in China.”
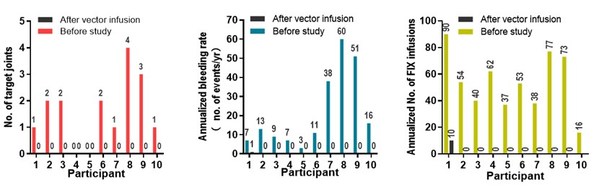
After vector infusion, BBM-H901 has brought significant clinical benefits to patients. The median annualized bleeding rate decreased from 12 times to 0, the median number of target joints decreased from 1.5 to 0, and the median number of FIX infusions decreased from 53.5 times to 0. In addition, BBM-H901 also demonstrated a good safety profile, with no Grade 3-4 adverse events (AE) reported over the follow-up period.
BBM-H901 has proved its safety and efficacy within the Chinese population, delivered a solution for the long-term treatment of hemophilia B and alleviated the occurrence of related complications for the betterment of patients. The clinical research of BBM-H901 is expected to provide a reliable basis and theoretical support for the clinical application of systematically administered gene therapy drugs in the future and is of important guiding significance for promoting the research and development and clinical transformation of gene therapy drugs in China.
About Hemophilia
Hemophilia, an inherited bleeding disorder, is mainly caused by mutations in the coagulation factor VIII or IX genes. Spontaneous bleeding may occur due to a significant reduction in coagulation factor VIII activity (FVIII:C, hemophilia A) or factor IX activity (FIX:C, hemophilia B) in patients. Repeated bleeding in joints and muscles may cause lifelong disability. Nowadays, prophylaxis and on-demand therapy with coagulation factor remain the standard clinical treatment for hemophilia. Patients need repeated injections of plasma-derived or recombinant coagulation factors throughout their life to maintain their coagulation function. As a result, the development of drugs that can cure hemophilia is a goal that scientists relentlessly pursue worldwide, and gene therapy has become a cutting-edge technology to cure hemophilia.
About Belief BioMed
Founded in 2016, Belief BioMed has become a globally leading company by being committed to providing innovative therapies with improved efficacy for monogenic disorder diseases, age-related degenerative diseases and certain malignant diseases through its AAV vector technology from early discovery to commercialization. The R&D and production strengths of Belief BioMed have been recognized by top investment institutions and enterprises. Belief BioMed has offices, research centers and manufacturing facilities in Shanghai, Hong Kong, Beijing and Suzhou China and North Carolina US.