Day 1 ASCO 2021 Roundup: Combating Lung Cancer, Hematologic Malignancies and Advanced Solid Tumors
The ASCO 2021 virtual annual meeting commenced on June 4th with several exciting presentations. Here is the Day 1 highlight of the conference replete with interesting talks handpicked by us.
New Avenues to Defeat Advanced Solid Tumors
Novartis’ TNO155, an Allosteric SHP2 Inhibitor Shows Early Promise
SHP2 is a nonreceptor protein tyrosine phosphatase essential for cell growth and differentiation. Inhibition of SHP2 leads to immunomodulatory effects in certain tumor microenvironment cells and helps mount antitumor immune responses. Once deemed ‘undruggable’, the oncogenic phosphatase has gradually become an attractive therapeutic target with the arrival of allosteric SHP2 inhibitors.
Novartis’ TNO155 is among the first generation of allosteric SHP2 inhibitors currently evaluated in trials. Dr. Irene Brana from the Vall d’Hebron University Hospital in Spain presented initial results from an ongoing dose finding study CTNO155X2101 that evaluated TNO155 in adults with solid tumors.
Results showed that TNO155 demonstrated favorable pharmacokinetic properties and demonstrated effective SHP2 inhibition at tolerated doses. The recommended dose of the candidate is not declared as of yet. The inhibitor has the potential to enhance antitumor activity when combined with KRAS G12C inhibitors, blockade of MAPK pathway feedback reactivation, and reduction of immunosuppressive signaling in the tumor microenvironment.
Cyteir Therapeutics’ Small Molecule Inhibitor of RAD51-Mediated DNA Repair Impresses in Phase1/2 Trial
Cancer cell growth is aided by homologous recombination (HR), a process that is crucial for repairing DNA double-strand breaks. HR inhibition causes the accumulation of unrepaired strands leading to tumor cell death. CYT-0851 is a first-in-class inhibitor of RAD51-mediated HR.
Dr. Ryan C Lynch of the University of Washington/Fred Hutchinson Cancer Research Center presented results of a Phase 1/2 trial that evaluated Cyteir Therapeutics’ CYT-0851 in patients with advanced hematologic and solid tumors.
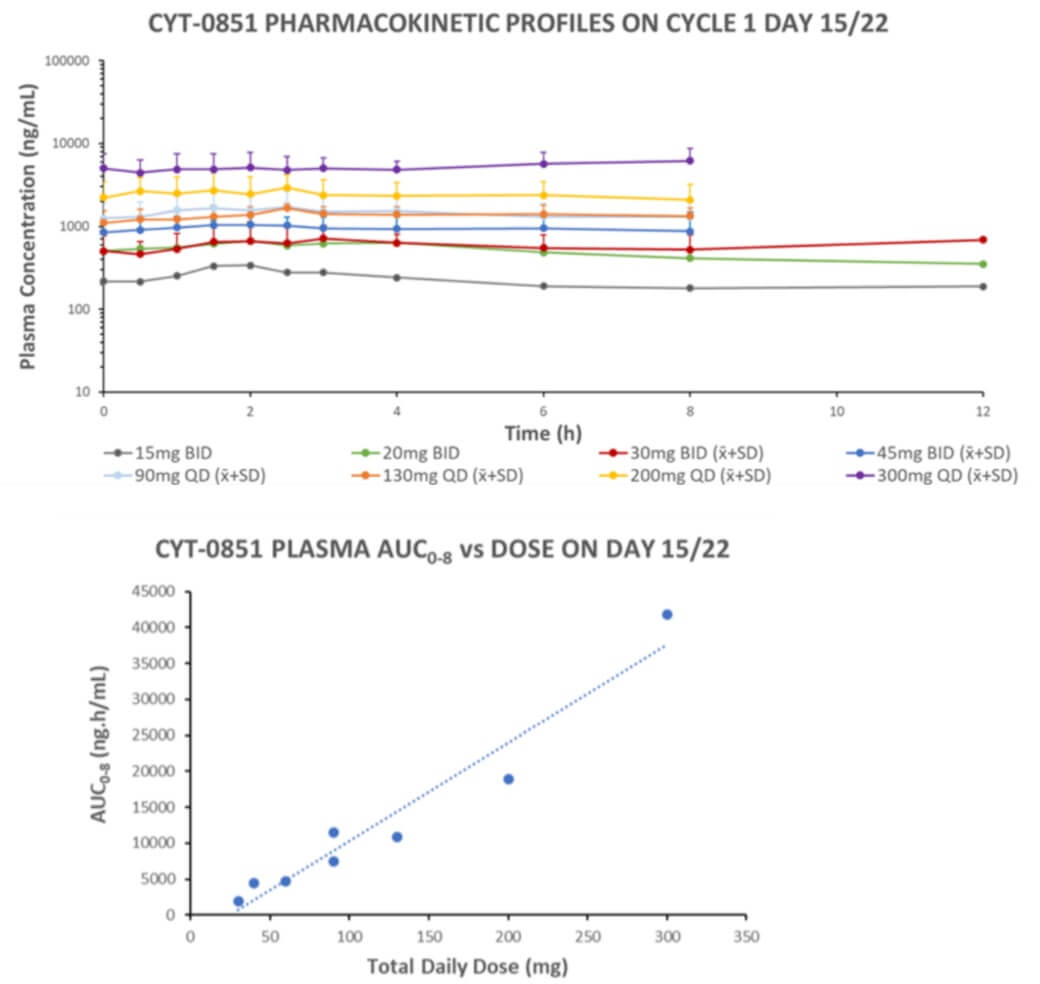
CYT-0851 was well tolerated, with linear pharmacokinetics, target-directed PD effects, and promising antitumor activity across different tumor types. CYT-0851 is the first DNA-damage repair (DDR) therapeutic with demonstrated clinical activity in both hematologic malignancies and solid tumors.
Beating NSCLC, the Most Common Type of Lung Cancer
Chemotherapy Plus Two Checkpoint Inhibitor Combo Provides Superior Long Term Protection
The combination of Opdivo, an anti-PD-1 antibody, and Yervoy, an anti-CTLA-4 antibody with two cycles of chemotherapy, has shown to improve objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) in patients with metastatic NSCLC. Due to the superior efficacy, this regimen has been approved in the US and EU as first line therapy for metastatic NSCLC.
Dr. Martin Rack, from the LungClinic in Germany, presented the 2 years’ minimum follow-up data for the Phase 3 CheckMate 9LA clinical trial. The trial compared the long-term efficacy of the combination therapy versus 4 cycles of chemotherapy. Results showed that the combo therapy had a higher OS (38%) compared to chemotherapy alone (26%), and median OS was longer by almost 6 months (15.8 months vs. 11 months). However, the patients had more adverse effects with combination therapy, but that arose mostly during the chemotherapy cycles. These results further support the use of this combo as first line therapy for metastatic NSCLC.
First FDA-approved KRAS Inhibitor Reports Topline Results in NSCLC
Amgen’s Lumakras made history after becoming the first ever FDA-approved therapy against KRAS. The KRAS inhibitor was approved for the treatment of NSCLC with a KRAS p.G12C mutation, and today we got updated data for the trial that led to its approval. Dr. Ferdinandos Skoulidis from the University of Texas MD Anderson Cancer Center presented the Phase 2 CodeBreaK100 trial results.
The trial enrolled more than 100 patients with locally advanced and metastatic NSCLC with verified KRAS p.G12C mutation. Patients received 960 mg of Lumakras daily. The data reported today was obtained at the median follow-up of 15.3 months. Results showed an ORR of 37%, and the median duration of response was more than 11 months. Importantly, more than 80% of patients achieved either complete response or partial and stable response. The treatment was safe, although 7.1% of patients discontinued the medication due to adverse effects.
A Phase 3, CodeBreaK200, clinical trial is underway comparing the efficacy of Lumakras versus docetaxel in KRAS p. G12C-mutated NSCLC.
Identification of a Subpopulation of EGFR Mutant Tumors that Respond to Rybrevant, Lazertinib Combo
Rybrevant is an antibody that targets both EGFR and MET receptors. It was recently FDA approved to treat NSCLC that are positive for EGFR exon 20 insertion mutation. Dr. Byoung Chul Cho presented the results of a combination regimen of Rybrevant plus lazertinib, a third-generation, mutant-selective EGFR tyrosine kinase inhibitor. The approach rests on double non-competitive inhibition of EGFR by both therapies. Rybrevant inhibits the extracellular part of EGFR, while lazertinib inhibits the intracellular part.
New therapies are necessary since the tumors with EGFR mutations can develop resistance to the first line therapy of osimertinib, and patients need to receive platinum-based chemotherapy. In the Phase 1 CHRYSALIS clinical trial, patients with NSCLC that acquired resistance to osimertinib were treated with the combination therapy in a dose-escalation regimen.
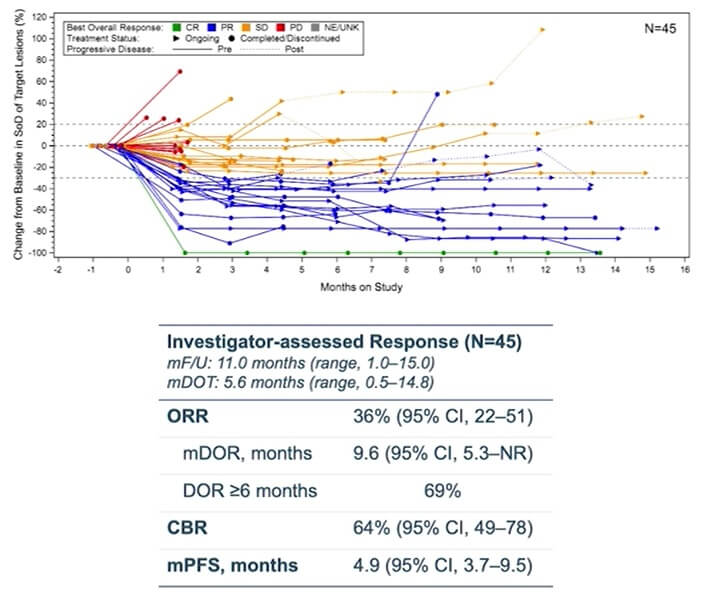 Results showed an ORR of 36% and a median duration of response of 9.6 months. Additionally, they identified that a subgroup of patients with high EGFR and MET expression were more likely to respond, which will be used as biomarkers in the Phase 1/1b clinical trial CHRYSALIS-2.
Results showed an ORR of 36% and a median duration of response of 9.6 months. Additionally, they identified that a subgroup of patients with high EGFR and MET expression were more likely to respond, which will be used as biomarkers in the Phase 1/1b clinical trial CHRYSALIS-2.
Hematologic Malignancies
Kite Pharma’s anti-CD19 CAR T Therapy Shines in Phase 2 ZUMA-3 Trial
Dr. Bijal Shah from the Moffitt Cancer Center in Florida presented the Phase 2 results of ZUMA-3, a clinical study for testing KTE-X19, a chimeric anti-CD19 CAR T-cell therapy in patients with relapsed/refractory B-cell Acute Lymphoblastic Leukemia (R/R B-ALL).
R/R B-ALL is an aggressive blood cancer with high rates of relapse and poor prognosis. Trial participants included adult patients with R/R B-ALL who had a high disease burden and received prior treatment of immunotherapy/stem cell transplants or other lines of treatment.
With a single dose of KTE-X19, most responded well with an overall complete remission rate of 56% and Cr/Cri rate of 71%. Responders had a median residual disease negativity of 97% and 31% had not yet reached median OS, suggesting long term protection. The toxicity profile was similar to Phase I data with largely reversible adverse events of mild pneumonia and neurotoxicity, most of which occurred in the initial days of infusion.
Responders with demonstrated higher peak CAR T-cell levels for about 15 days with some association with low response and/or mild adverse events. In conclusion, KTE-X19 ticks off the boxes in efficacy, safety, and scaled-up manufacturing as a promising therapy for adult R/R B-ALL patients.
Study of Maintenance ixazomib after alloHCT for High Risk Multiple Myeloma Stopped Early
Dr. Taiga Nishihori from Moffitt Cancer Center discussed the intricate challenges in clinical trials for high-risk multiple myeloma (MM). Allogenic hematopoietic cell transplantation (alloHCT) is a potential therapy for MM. Patients who receive transplant from a HLA-matched sibling show some adverse reactions and toxicities due to immunomodulatory agents. Hence, pre-conditioning with proteasome inhibitor maintenance with other drugs is considered to improve response.
In a Phase 2 study, ixazomib was evaluated for its role in the maintenance of alloHCT. Of 57 enrolled, 52 patients received preconditioning and transplants. In a double-blind RCT, half of them were administered with ixazomib and 22 placebo for maintenance therapy. The study was delayed due to toxicity issues from the conditioning regimen, transplant-related mortality, and other issues and hence prematurely closed.
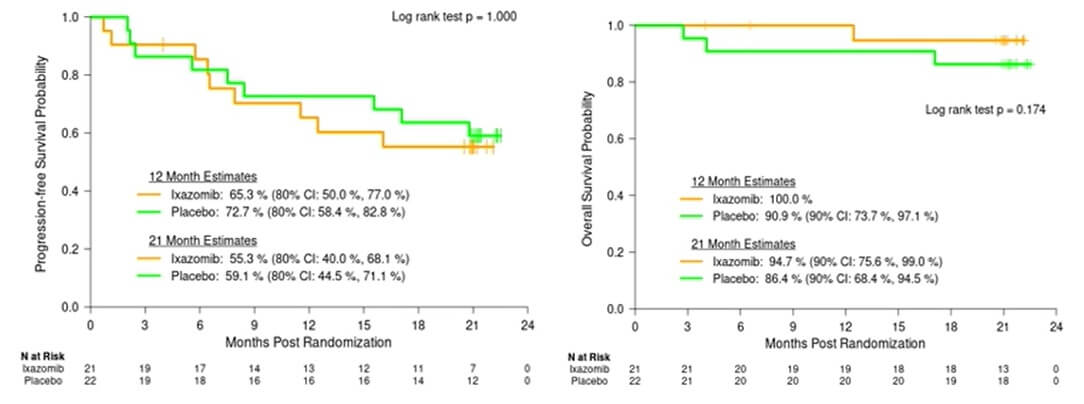
After 21 months, both groups had similar PFS, OS, and incidences of GVHD (graft versus host disease), suggesting no clinical impact of ixazomib. However, a pre-conditioning regimen of Flu/Mel/Bort is safe for MM.
By Rajaneesh K. Gopinath, Daniel Ojeda and Sahana Shankar
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com








