Harvard’s Soft Robotic Wearable Device Helps ALS Patients Regain Arm Mobility
In a collaborative study conducted by Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS) and Massachusetts General Hospital (MGH), researchers have developed a soft robotic wearable that is capable of significantly assisting the movement of the upper arm and shoulder in people with Amyotrophic Lateral Sclerosis (ALS, also known as Lou Gehrig’s disease), helping them to partially regain arm mobility.
Led by Dr. Conor J. Walsh, the Paul A. Maeder Professor of Engineering and Applied Sciences at SEAS, the research team reported their findings in an article published in the February 1 issue of the journal Science Translational Medicine.
Related article: Insilico Medicine Pinpoints New Targets for ALS With AI
A Devastating Neurodegenerative Disease
In patients with ALS, nerve cells in the brain and spinal cord responsible for voluntary muscle movement (motor neurons) are progressively destroyed, leading to muscle weakness and atrophy. The progression of ALS differs between each patient, but the disease typically advances rapidly within 2 to 5 years, causing irreversible loss of ability to stand, move, speak, swallow, and even breathe.
It is estimated that there are more than 30,000 Americans who may be living with ALS at any given time. Given that new disease-modifying treatments that will prolong life expectancy are emerging, there is an imperative need for new tools that can improve patients’ independence with everyday activities.
Lightweight, Wearable Device that Restores Mobility in ALS Patients
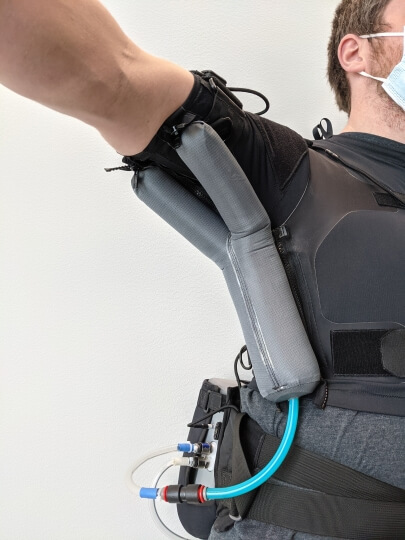
The robotic wearable device for ALS patients developed by the Harvard team is soft, fabric-based, and powered cordlessly by a battery. It is also lightweight which adds only 150 grams of weight to each upper limb of the wearer.
Dr. Tommaso Proietti, the paper’s first author, describes it as a shirt with some inflatable balloon actuators under the armpit. With the aid of pressurized balloons, the wearer is able to overcome gravity and move their upper arm and shoulder. The device is also relatively safe to use, without posing risk of injury from the robot even when it fails.
In order to assist people living with ALS, the team developed a sensor system that detects residual movement in the arm and calibrates the balloon actuator in order to enable natural movement in the arm. The researchers also recruited 10 ALS patients with different degrees of neuromuscular impairment for testing to evaluate the effectiveness of this wearable device in extending or restoring their ability of body movement and quality of life.
The soft robotic wearable is easy to use. It only takes 30 seconds for the calibration process to detect each wearer’s unique level of mobility and strength. Also, wearers can learn how to use the device within 15 minutes. The team found in their study that the soft robotic wearable improved participants’ range of motion, reduced muscle fatigue, and increased performance of tasks like holding or reaching for objects.
Hope for Extending Application to Severe Patients
The current prototype developed for ALS was only capable of functioning on study participants who still had some residual movements in their shoulder area. Team members are testing possible versions of assistive wearables that could be controlled by neural signals in the brain. They hope that one day such a device may help severe ALS patients who no longer have any residual muscle activity.
The team is eager to develop robotic wearable devices that function like apparel and be comfortable to wear for long periods of time, bringing improvements to the lives of ALS patients. It is anticipated that sufficient progress of the research will be made in the future, allowing the conversion of this new technology into a commercial product.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com








