India Approves Oral COVID-19 Drug for Emergency Use to Abate Oxygen Crisis
The second COVID-19 wave that started in February has left India with a severe oxygen scarcity for hospitalized patients. With more than 3.7 million active cases reported, the country is reeling with multiple setbacks at once. This includes the rise of new variants, vaccine shortages, and the spread of COVID-19 from big cities to even the remotest villages. While global nations have lent a helping hand to manage the COVID crisis, India still strives for long-term solutions to withstand the burden of its huge population.
In April, the Drugs Controller General of India (DCGI) approved Zydus Cadila’s Virafin for emergency use in patients with moderate COVID-19 infection. The Pegylated Interferon alpha-2b administered as a single dose subcutaneous injection, was greenlighted as an alternative to remdesivir. Now, the drug regulator has approved an anti-COVID drug that can be administered orally.
On May 8th, the DCGI approved 2-deoxy-D-glucose (2-DG) as an adjunct therapy for emergency use in patients with moderate to severe COVID-19. “Clinical trial results have shown that the molecule helps in faster recovery of hospitalized patients and reduces supplemental oxygen dependence,” the government of India said in an official statement.
2-deoxy-D-glucose
2-DG is an investigational drug currently evaluated as an anticancer and antiviral agent. It is a synthetic glucose analog in which the 2-hydroxyl group is replaced by hydrogen.
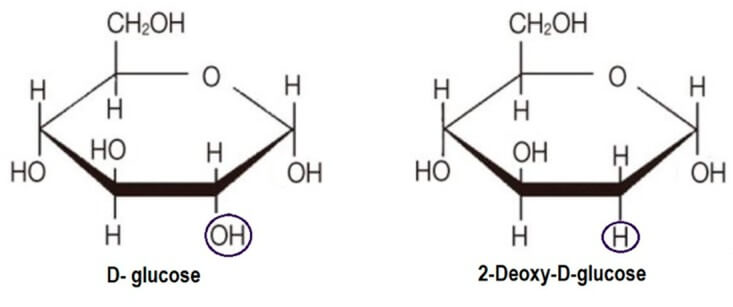
Molecular Structure of D-glucose and 2-DG (Pajak, et al.)
2-DG was produced by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a lab under the Defence Research and Development Organization (DRDO), in collaboration with Hyderabad-based Dr. Reddy’s Laboratories. According to the statement, 2-DG can be easily produced and made available in plenty in the country.
The seeds of the approval were sown during the first wave of the pandemic in 2020 when INMAS-DRDO scientists, along with the Centre for Cellular and Molecular Biology (CCMB), investigated the benefits of 2-DG in the lab. They had found that it inhibited the growth of the SARS-CoV-2 virus.
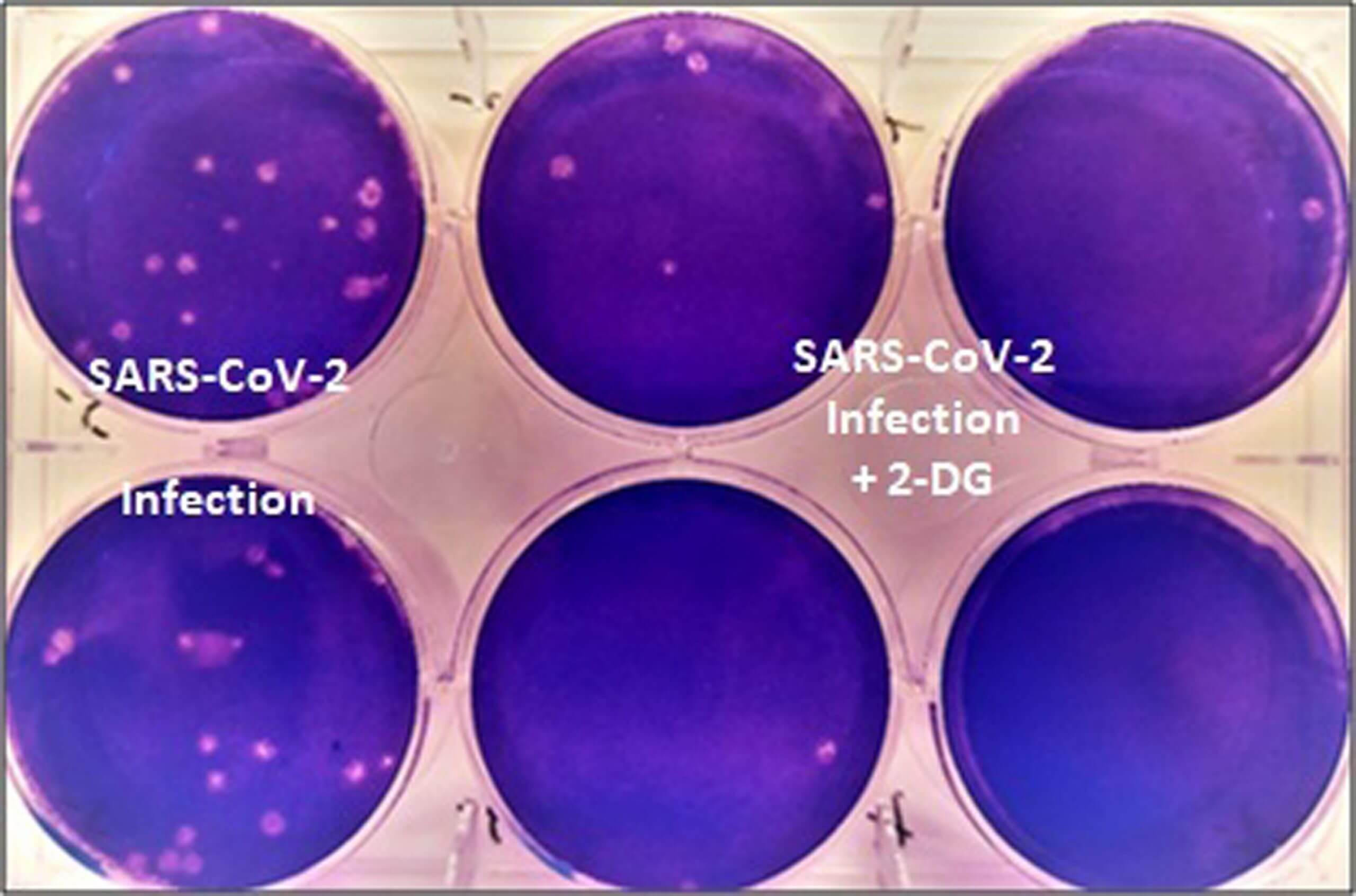 Image Courtesy: Press Information Bureau, Government of India
Image Courtesy: Press Information Bureau, Government of India
The subsequent Phase II trials conducted on 110 patients demonstrated that the drug was safe and significantly improved patient recovery. Besides, it showed a faster symptomatic cure than the standard of care ( SoC) on various endpoints. The Phase-III clinical trial was conducted on 220 patients at 27 COVID hospitals across the country in Delhi, Uttar Pradesh, West Bengal, Gujarat, Rajasthan, Maharashtra, Andhra Pradesh, Telangana, Karnataka, and Tamil Nadu.
“In 2-DG arm, a significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs. 31%) by Day-3 in comparison to SoC, indicating an early relief from Oxygen therapy/dependence,” the statement read.
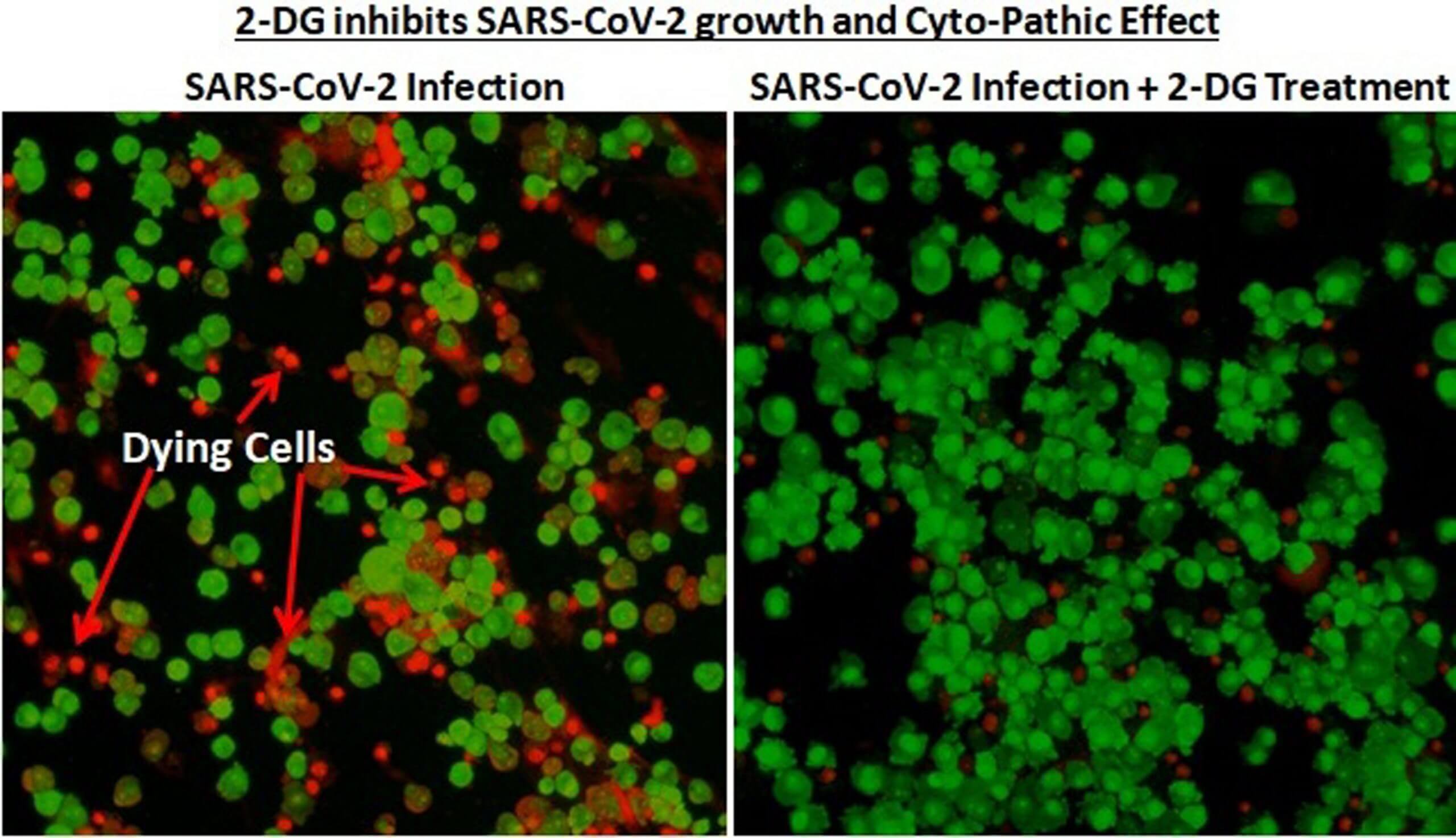 Image Courtesy: Press Information Bureau, Government of India
Image Courtesy: Press Information Bureau, Government of India
Mode of Action
The drug, which is in powder form, will be available in sachets. It can be administered in patients orally by dissolving in water. “It accumulates in the virus-infected cells and prevents virus growth by stopping viral synthesis and energy production. Its selective accumulation in virally infected cells makes this drug unique,” said the statement.
“In the ongoing second COVID-19 wave, a large number of patients are facing severe oxygen dependency and need hospitalization. The drug is expected to save precious lives due to the mechanism of operation of the drug in infected cells. This also reduces the hospital stay of COVID-19 patients,” it added.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









