Janssen’s Multiple Sclerosis Drug Snags FDA Win after Besting Sanofi’s Blockbuster in Phase 3
Multiple sclerosis (MS) affects 2.3 million people globally, with 1 million in the US alone. It occurs when our immune system attacks the myelin sheath, a fatty substance that insulates our nerve fibers in the brain and spinal cord. The demyelination affects quick and effective neuronal communication, thereby disabling the central nervous system. Vision problems, muscle weakness, numb sensations, and cognitive issues are some of the common symptoms.
Relapse is often the most significant challenge, with Relapsing-remitting MS (RRMS) being the predominant form, Other forms include primary progressive MS (PPMS), secondary progressive MS (SPMS), and clinically isolated syndrome (CIS) that denote patients who exhibit MS-like symptoms but isn’t diagnosed yet.
A New Entrant in an Already Crowded Market
On March 19th, the USFDA approved yet another MS drug—Janssen’s Ponvory (ponesimod). The oral, sphingosine-1-phosphate receptor 1 (S1P1) modulator received the agency’s nod for treating relapsing forms of the disease, RRMS, SPMS, and CIS. Ponvory has now entered a market crowded with pharma giants, namely, Novartis, Biogen, Roche, Bayer, Pfizer, Merck, Sanofi, Teva, and GSK (See Table 1 & 2).
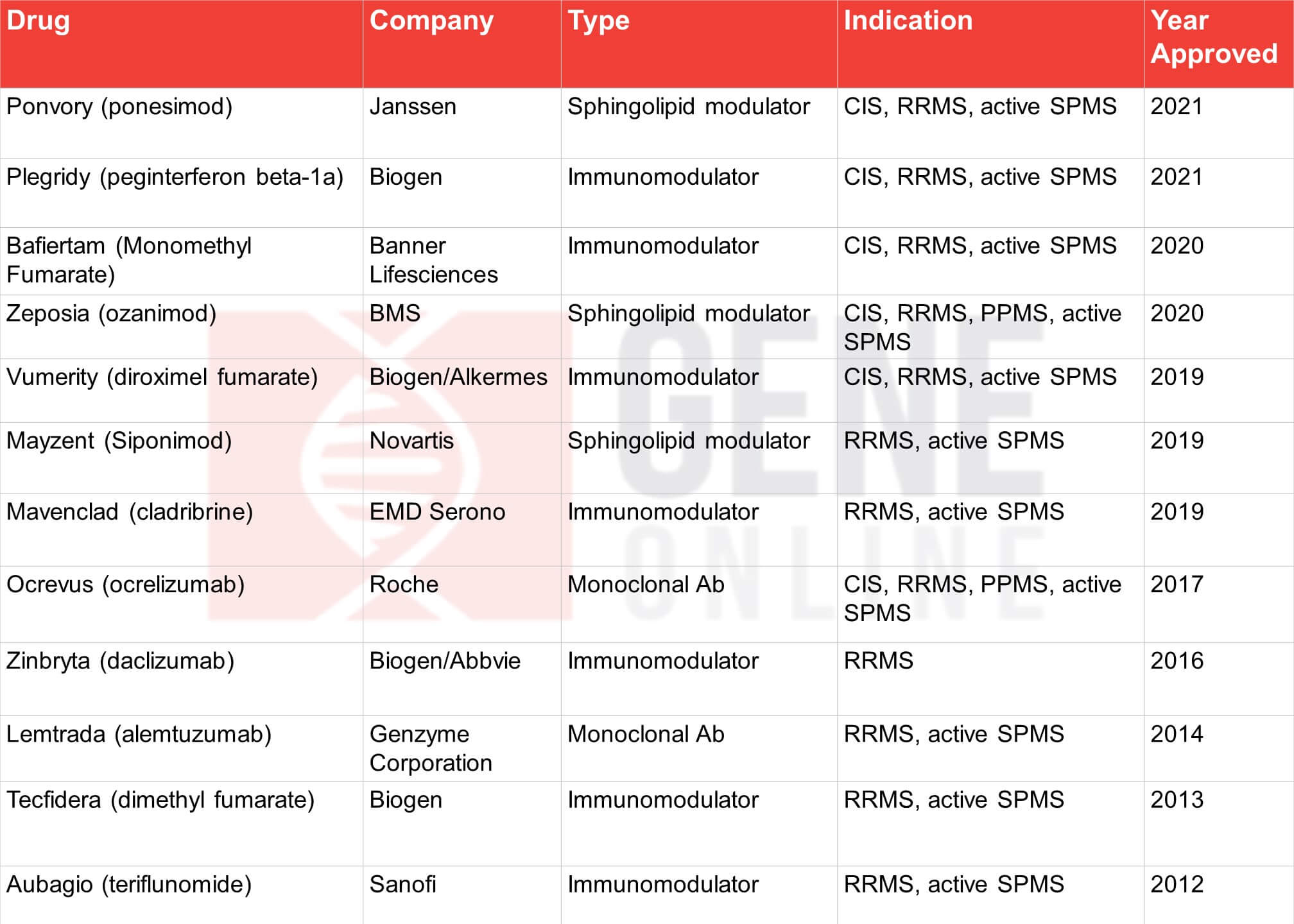 Table 1: FDA approved MS drugs since 2010
Table 1: FDA approved MS drugs since 2010
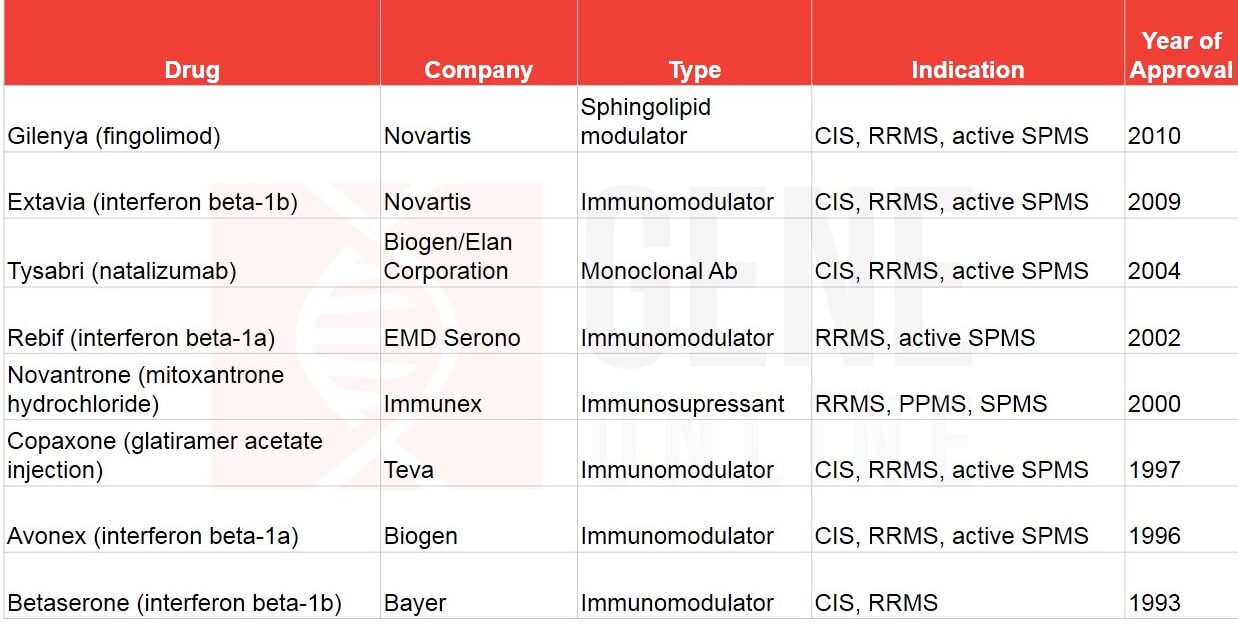 Table 2: FDA approved MS drugs on or before 2010
Table 2: FDA approved MS drugs on or before 2010
“Every person with multiple sclerosis is affected differently, given variability in both the underlying disease and emerging symptoms. Continued innovation in this space is critical, and we’re committed to meeting patients’ evolving healthcare needs,” said Mathai Mammen, M.D., Ph.D., Global Head, Janssen Research & Development, Johnson & Johnson. “We are proud to offer PONVORY as a valuable new option for people with MS that may help them gain better control of their disease.”
Strong Trial Data Surpassing Established Therapy
The FDA approval is an outcome of positive results for Ponvory, which was pitted against Sanofi’s billion-dollar drug, Aubagio (teriflunomide), in Phase 3, OPTIMUM trial comprising of 1,133 participants. Superior showing in annualized relapse rate (ARR), a measure of the average number of relapses that trial patients experience in a year, was the primary endpoint.
Results from the two-year study period showed that 20mg of Ponvory significantly reduced annual relapses by 30.5% as compared to 14mg of Aubagio. Besides, 71% of patients treated with Ponvory had no confirmed relapses as opposed to Aubagio’s 63%. Ponvory also delayed disability progression and registered benefits over Aubagio in standard assessments of MS pathology and disease burden as measured by MRI scanning of the brain and spinal cord. The drug was well-tolerated over multiple clinical studies spanning more than 10 years. Upper respiratory infection, hepatic transaminase elevation, and hypertension were some commonly observed side effects.
“In the pivotal study, ponesimod demonstrated superior clinical efficacy in reducing annual relapses and MRI activity compared against teriflunomide, another oral MS therapy. Those results, combined with a favorable side effect profile, make ponesimod a useful treatment option for people with relapsing MS,” said Robert J. Fox, M.D., Staff Neurologist at Cleveland Clinic and member of the ponesimod Advisory Board.
Ponvory is the first and only FDA-approved, oral, disease modifying therapy studied in a head-to-head trial against an established oral comparator drug, Janssen said in a statement.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com







