Taiwanese Startup, Micronbrane Aims to Improve Detection of Microbial Pathogens
(Partner Content)
Bloodstream pathogens are identified through blood cultures conventionally, and this process can take up to 2-3 days. Recent advances in nucleotide sequencing technologies provide a faster and more accurate solution for pathogen detection. However, host DNA interference is still a major obstacle during blood sample processing prior to sequencing. Micronbrane Medical, a new startup based in Taiwan, aspires to overcome the hurdle and provide a complete solution for bloodstream pathogen detection.
Human DNA Removal is Key for a Better Diagnostic
The removal of human DNA from blood samples is a challenging and critical step as it significantly impacts the accuracy of the test result.
“It is challenging for medical professionals to remove genetic interference from human DNA from blood samples before genome sequencing,” noted Dr. Mengchu Wu, Chairman of Micronbrane Medical.

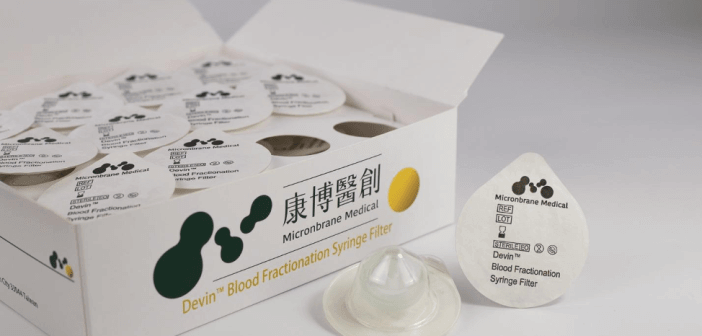
Micronbrane Medical provides the solution with two products: Devin® and PaRTI-Seq® (Pathogen Real-Time Identification by Sequencing).
Devin® membrane uses proprietary filter technology to separate white blood cells of whole blood within 3 to 5 minutes. The membrane captures white blood cells specifically while allowing other blood components to flow through. Pathogen-enriched samples are achieved through the removal of host white blood cells. This product is currently for research use only (RUO) but soon will be applied in the clinical setting as part of an in vitro diagnostic device (IVD).
PaRTI-Seq® technology combines the latest third-generation sequencing (Nanopore Sequencing) and proprietary analytical methods in order to provide test results within 22 hours. The platform streamlines the workflow of blood pathogen detection in order to provide faster and more accurate detection. The technical and clinical validation of the PaRTI-Seq® system is expected to be completed in the first quarter of 2021 before offering it for clinical use in the market.
Taiwan’s Immense Potential in Clinical Microbiology Diagnosis
Clinical microbiology diagnosis is relatively new in the field of precision medicine, as compared to cancer and antenatal examination. According to chairman Dr. Wu, biotech companies in Taiwan are showing great promise in this domain. With patented host depletion technology and proprietary analytical methods, Micronbrane Medical has competitive advantages over international competitors in providing solutions for bloodstream pathogen detection.
For Partnership and Free Sample – Devin® Offering: https://reurl.cc/7o1oxy
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









