New COVID-19 Saliva Test Bags FDA Emergency Approval
By Ruchi Jhonsa, Ph.D.
It is not easy to collect COVID-19 samples, as it seems. Currently, sample collection for COVID-19 depends entirely on professional healthcare workers inserting a swab all the way back into the patient’s nasal cavity and gently scraping the tissue to collect the material. If this is not performed well, it can result in false-negatives. Moreover, the current sample collection method demands qualified healthcare workers to wear fresh gloves and other protective gear, which are not always available in plenty and puts them at risk of acquiring the infection themselves. Justin Bellante, CEO of BioIQ rightly pointed, “When a physician uses a nasal swab, they have to put that so far back into the nostril that many people sneeze. They get all that fluid on the personal protective equipment of the physician.” This demands for sample collection methods, which are safe and reduce the burden on healthcare workers.
A sample collection approach developed by clinical genomics researchers at Rutgers University in collaboration with Spectrum Solutions and Accurate Diagnostic Labs solves many of the above problems. The approach uses saliva secretions from the mouth of suspected coronavirus patients to detect viral load making it easy for healthcare workers to collect. The spit method correlated well with the swab collection method according to the validation results provided to the FDA, which showed similar results for 60 swab and saliva samples. This test got emergency FDA approval on April 11th. With this approval, the risk of health care professionals can be significantly reduced as well as precious personal protective equipment that can be reserved for use in patient care instead of sample collection.
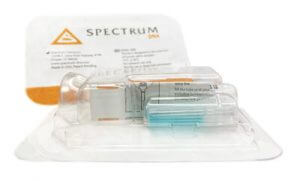 (Photo credits: Leslie Titus Bryant, Director of Marketing & Brand at Spectrum Solutions)
(Photo credits: Leslie Titus Bryant, Director of Marketing & Brand at Spectrum Solutions)
Calling it “innovation under pressure,” President Trump praised this new method of testing as it can substantially reduce the burden on healthcare workers. For now, the test can only be availed through RWJBarnabas Health network, New Jersey’s most comprehensive health care system, which includes Robert Wood Johnson University Hospital, University Hospital in Newark and several county health departments. Rutgers is also prepared to provide drive-thru testing in partnership with the Middlesex County government and RWJBarnabas Health at 33 Kilmer Road, Edison, New Jersey. This is good news for the state, which has the second-highest number of coronavirus cases in the country.
The spit collection method has already been tested before however, not for measuring the viral load. It is frequently used by genealogy companies like 23&me that collect patient’s spit in a tube for DNA analysis. Pointing at the simplicity of this new collection method, Rupen Patel, CEO of Accurate Diagnostic Labs said, “Now imagine – you pull up, you roll down your window, they give you the collection device, it takes seconds to spit into it. You put the cap on, which releases the preservation solution into the vial. You wipe it with a disposable alcohol pad, and you hand it back to the person at the window and you go off on your way. Twenty-four to 48 hours later, you get your test results. All this can happen while mitigating exposure and using best efforts to reduce the use of valuable PPE (Personal Protective Equipment).”
Soon after the FDA announcement, the research team was contacted by chief executive officers of some of the world’s largest life sciences companies that are involved in COVID-19 testing. “I have spoken with these companies’ leadership to not only share knowledge but to create opportunities for continuing to help innovate during this crisis,” Brooks said. “We will work closely with these new partners, the FDA and the White House task force to leverage everything Rutgers has to offer to not only help our community but also make a global impact.”
Related Article: United States To Procure Test Kits from COVID-19 Curbing South Korea
References
- https://www.rutgers.edu/news/new-rutgers-saliva-test-coronavirus-gets-fda-approval
- https://spectrumsolution.com/rutgers-research-lab-using-new-sdna-saliva-collection-device-to-test-for-covid-19/
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









