New Frontiers in Therapeutics
In the “New Frontiers in Therapeutics session” of BIO Asia 2020, speakers shared their thoughts on topics of cell and gene therapy, manufacturing achievements and challenges, and regulatory pathways in different countries.
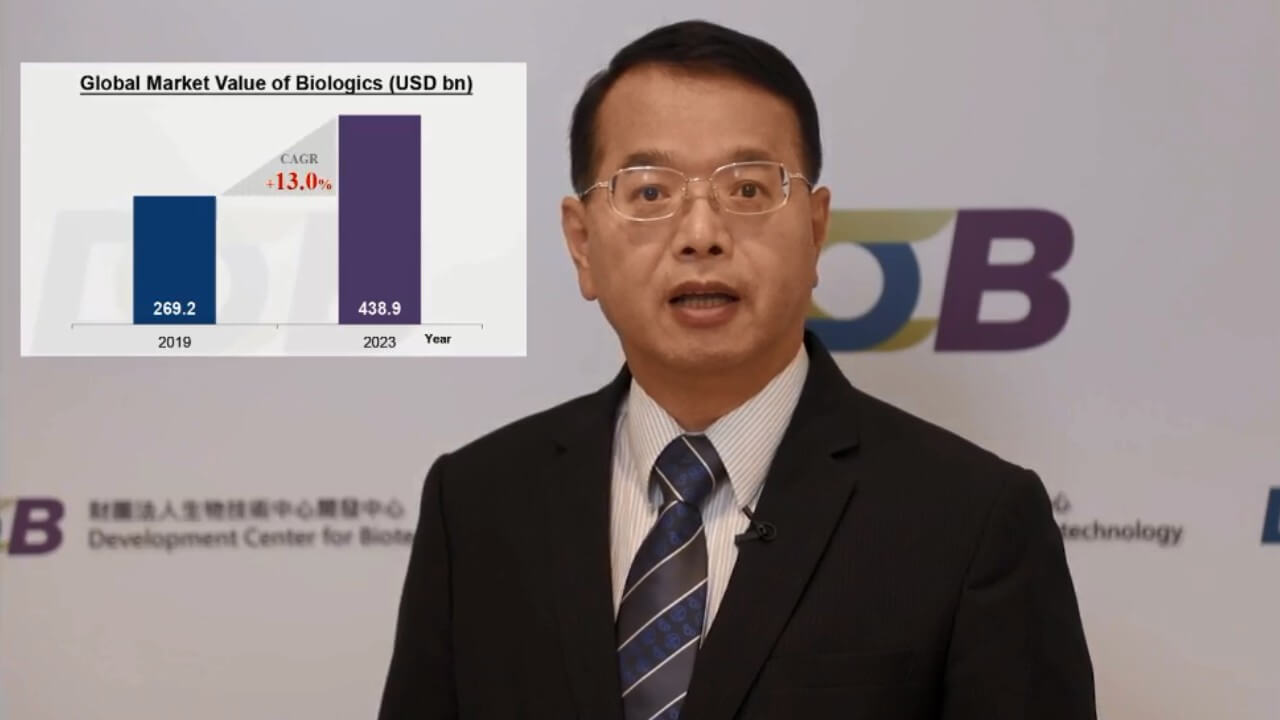
The opening remarks were delivered by Dr. Chung-Hsiun Wu, President, Development Center for Biotechnology (DCB). He mentioned that the market value of global biologics in 2019 was 269.3 billion. It is expected to reach 438.9 billion in 2023, at a compound growth of 13%. Furthermore, the global market value of cell therapy is expected to grow from 9 billion to 20.2 billion with a compound growth of 22.4%. Therefore, it is evident that we will find more business opportunities created by the development of innovative biotherapies, new platforms, and product manufacturing.
 Dr. Chung-Hsiun Wu, President, Development Center for Biotechnology (DCB)
Dr. Chung-Hsiun Wu, President, Development Center for Biotechnology (DCB)
Biotherapies: Challenges and Opportunities
Toru Seo, Senior Director and APAC head of Scout and Evaluation, began by highlighting Pfizer’s approach to identifying promising frontiers in drug discovery. Instead of going after individual diseases, the company favors exploring underlying mechanisms that span multiple diseases. Three scientific areas were described in more detail: repeat expansion disorders, senescence, and DNA damage response. He stressed that focusing on broader mechanisms would open avenues for targeting various indications simultaneously.

His presentation then switched to Pfizer’s oncology research efforts, specifically redirected T cell killing via bispecific antibodies (BiS). These engineered antibodies can direct and recruit T cells to tumors via Fab regions, while also bringing macrophages via the Fc region (tri-functional mAb). Dr. Seo also noted that the number of clinical trials and investments in this area is steadily growing over the past decade.
In the second half of his talk, he remarked on various modalities showing great clinical and commercial promise. They include gene therapy, cell therapy (CAR-T), TCR like CAR targeting intracellular tumor antigen, PROTACs (PROteolysis TArgeting Chimeras), and nanobodies.
Although the use of the latest digital tools for drug discovery is an active area of development in Pfizer, there are several challenges in bringing therapies to the market. Among them are long-term efficacy and safety of medicines, identification of right patients for precision medicine, and subsequent commercialization framework to make them affordable and several regulatory hurdles and supply chain issues. Dr. Seo concluded that building strategic partnerships, collaborations, and licensing agreements between various companies is the solution.
New Discoveries, Advances, and Technologies in Biologics
John Yu, Distinguished Chair Professor, and Director, Chang Gung Memorial Hospital, focused on the recent advances in the development of anticancer biologics. Since cancer cells display specific biomarkers on their surface, they can be selectively targeted. To achieve that, a recently patented platform for the development of Fn cancer-targeting peptides (Fn-CTP) is employed. It leverages a phage display library and computer-aided methods for optimization of the final complex structure. Resulting peptides are chemically conjugated to various delivery systems, including bispecific antibodies, RNA-based therapies (miRNA/siRNA), toxins, liposome drug, and CAR-T, among others.

He also mentioned two applications of the FnCTP platform in more detail. While bispecific antibodies only target a particular cancer type, conjugating them to FnCTP would allow us to target a broad cancer spectrum via activation of T cell-mediated cytotoxicity. The second conjugation method is with a toxin-drug, done in collaboration with Dr. Ira Pastan from NIH. The co-developed therapeutics can target cancer stem cells, have demonstrated nanomolar IC50 values, and are progressing into clinical trials for evaluation.
Lastly, he highlighted the development of Cell Therapy Center, its state-of-the-art good tissue practice (GTP) facility, and the cleanroom with the latest QC and surveillance and safety protocols. The facility is used to produce targeted Natural Killer cells such as cord blood NK, and iPSC derived NK via a unique 2-step NK cell expansion protocol, yielding highly pure, viable, and cytotoxic cells as compared to other methods.
A NEW Toolbox for Oncology Therapies
Wei-Kuang Chi, Vice President, R&D, Development Center for Biotechnology (DCB), spoke about their four proprietary platforms for oncology therapies. The first is a site-specific antibody-drug-conjugate (ADC) platform that uses tri-mannosyl (TM) sugars of the antibody to attach either single or dual payloads with a homogeneous drug to antibody ratio of 2 or 4. Dr. Chi showed DCB’s work on five targets and ten different antibodies in xenograft models. Specifically, TM trastuzumab ADC showed promising results against breast and gastric tumors. Anti-globo H ADC showed antitumor activity against globo H breast cancer cell line, and anti-MSLN (mesothelin) ADC showed potency in both pancreatic and ovarian cancer models.

Another proprietary platform described was an immuno-oncology (I/O) Toolbox. It includes novel anti-PD-L1 mAb, which in their competitive binding assay, showed that its epitopes are not overlapping with commercially available antibodies. Besides, it includes anti-CSF-1R (colony-stimulating factor 1 receptor) mAb and anti-TIM-3 (T-cell immunoglobulin mucin-3), a second wave immune checkpoint) mAb, both of which showed specificity against their targets and appropriate and potent cellular responses in different functional studies.
The third, DCB’s CAR-T platform, has several key advances in its development and was noted to increase CAR-T cell growth rate, with expansion and potencies of cells higher than industry benchmarks. The final toolbox that Dr. Chi mentioned was PROTAC (PROteolysis TArgeting Chimeras), which is used to target KRAS in a non-small cell lung cancer model.
Managing Cell Therapy Product Manufacturing and Quality Control
Kunihiko Suzuki, Vice President, Member of the Board (MDNT)/Vice Chairman, Member of the Board (FIRM), described the two segments of MEDINET, a contract cell manufacturing business, and regenerative medicinal product business. The first, which operates akin to a CMO/CDMO, is regulated in Japan by two main statutes: The Act on the Safety of Regenerative Medicine and The Act on Pharmaceuticals and Medical Devices.

Within this manufacturing business division, various cell therapies have been produced for medical institutions treating cancer patients over the last twenty years. A cell processing facility at Shinagawa that is fully compliant with various regulations in Japan and a licensing agreement with Taiwanese biopharmaceutical company Medigen Biotechnology Corp. were highlighted to illustrate that MEDINET is well-positioned in the regenerative medicine market.
The second topic that he covered was the findings and experiences within the value chain of cell manufacturing. The first consideration is which specific regulatory framework cell manufacturing falls under (products vs. medical care) and is dependent on the country. The second is manufacturing by autologous or allogeneic cell sources, which often requires a lot of interactions with clients, and discussions about cell characterization, scale, validation, and QC/QA.
The third challenge is in specifics of logistics/transportation of cell therapies such as sample preservation by freezing. Technology Transfer is another important consideration for successful and timely commercialization of cell therapies, due to the lengthy adoption of SOPs for CMOs and potentially high upfront costs. The reduction of production costs via automatization, streamlining QA/QC procedures, and other means is also vital to successful business operations in cell therapy space.
Regulatory Framework of Cell Storage and Processing Industries in Cell Therapy — From Taiwan to Global
Jarret Su, Head of Healthcare and Life Science, KPMG in Taiwan, began his presentation by describing key regulatory milestones in cell therapy space across the world over the last decades and the approved cell therapy products in key markets, noting the increasing number of clinical trials, and lucrative capitalization projections in this space.

He then introduced the differences in patent application processes between the US and China, and the major sources of law in various regions around the world, such as 21st Century Cares Act in the US, RM Act and PMD Act in Japan and a similar regulatory system in Taiwan, where medicinal products and medical technology are regulated in parallel. In the latter bracket, six autologous cell therapies were described: peripheral blood stem cell transplantation, immune cell therapy, adipose stem cell transplantation, fibroblast transplantation, bone marrow mesenchymal stem cells, and chondrocyte transplantation.
Dr. Su concluded by describing the four major demands in the cell therapy industry, such as automation in cell and gene therapy manufacturing, high standard cell foundry, low-temperature logistics, and big data cloud monitoring. He concluded with exciting business opportunities around regenerative medicine, like consumables, equipment, various adjacent services, and innovative drug applications.
Editor: Rajaneesh K. Gopinath, Ph.D.
Related Article: A New Paradigm in Biotech Innovation and Investment
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com








