Novartis Announces Failure of Ruxolitinib Trial in Severe COVID-19
On December 14th, Novartis announced that its 29-day, Phase III study did not meet its primary endpoint. Initial data show no statistically significant reduction in the proportion of COVID-19 patients who experienced death, respiratory failure requiring mechanical ventilation, or admission to the intensive care unit (ICU). The trial also did not meet its secondary and exploratory endpoints, including mortality rate.
RUXCOVID Trial
RUXCOVID (NCT04362137) is a Phase III, multicenter, randomized, double-blind study that evaluated the safety and efficacy of ruxolitinib plus standard of care (SoC) therapy compared to placebo plus SoC therapy in patients aged 12 and above hospitalized for COVID-19 and not intubated or receiving ICU care prior to randomization. The study has enrolled 432 patients globally.
The composite primary endpoint is the percentage of patients who die, require mechanical ventilation, or require admission to ICU by Day 29. Secondary endpoints include various efficacy assessments including evaluation of clinical status using a 9-point ordinal scale; ICU stay, supplemental oxygen, invasive mechanical ventilation; the proportion of patients without oxygen therapy (O2 saturation of ≥94%).
Eligible patients were randomized 2:1 to receive 5mg of ruxolitinib orally twice per day (BID) or oral-matching placebo for a total of 14 days. Study treatment was given in combination with SoC therapy, according to the investigator’s clinical judgment. After 2 weeks of therapy, should clinical signs or symptoms not improve or worsen, and the potential benefit outweighs the potential risks, patients were eligible for another 14 days of study therapy. In total, patients are followed on study for 29 days post-randomization.
Ruxolitinib
Ruxolitinib is an oral inhibitor of JAK 1 and JAK 2 tyrosine kinases. It is approved under the trade name Jakavi® in Europe and other regions and countries for the treatment of adult patients with polycythemia vera (PV) who are resistant to or intolerant of hydroxyurea and for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (MF), post-polycythemia vera MF or post-essential thrombocythemia MF. Novartis licensed ruxolitinib from Incyte Corporation for development and commercialization outside the US.
Measuring Success for a Repurposed Drug Trial
The average success rate for a clinical trial in the field of infectious diseases is 25.2%. Despite this high rate of success (in this case, meaning FDA approval), there is still the issue of whether or not the drug will succeed in the market. This success rate also does not account for pandemics as they are both rare and extreme events.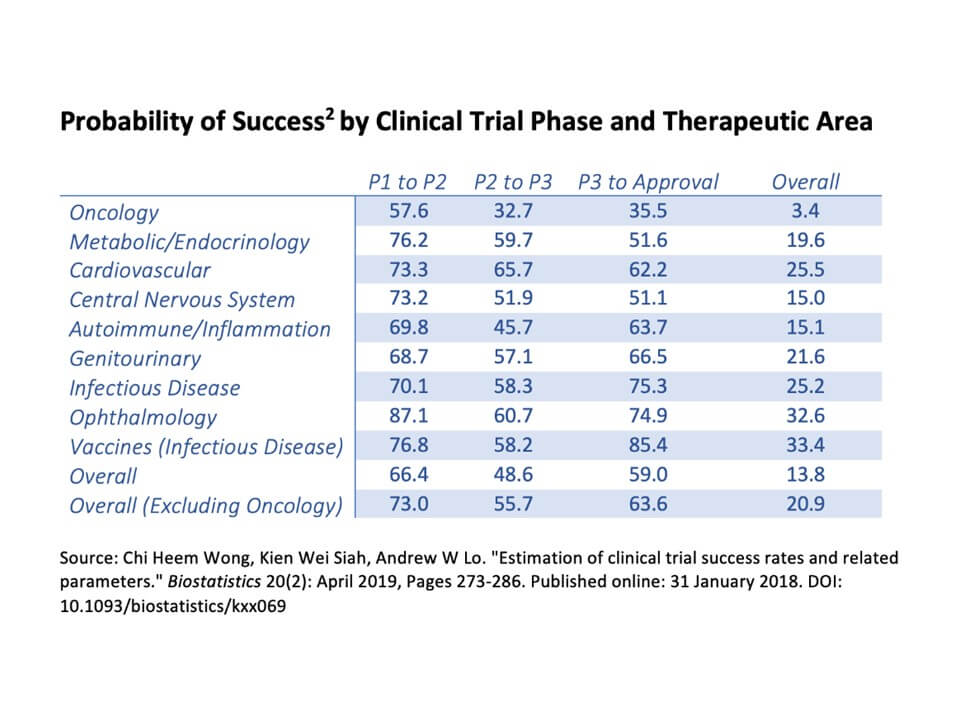 However, these data only account for first-time drug trials rather than a trial for a repurposed drug. While the above chart can help give a ballpark figure, a more accurate comparison for the RUXCOVID trial would be the trial for Dexamethansone’s use for hospitalized COVID-19 patients. While this clinical trial was successful, dexamethasone has been around since 1961 and has had ample time to be very well understood in its behavior within the human body.
However, these data only account for first-time drug trials rather than a trial for a repurposed drug. While the above chart can help give a ballpark figure, a more accurate comparison for the RUXCOVID trial would be the trial for Dexamethansone’s use for hospitalized COVID-19 patients. While this clinical trial was successful, dexamethasone has been around since 1961 and has had ample time to be very well understood in its behavior within the human body.
While a variety of COVID-19 treatments are under development, only a few might achieve regulatory approval. As a companion drug, repurposed drugs might only have limited utility. Drugs such as Dexamethasone have become a recognized alleviator of COVID-19 symptoms and would undoubtedly be more affordable than a new drug. Secondly, Ruxotinilib was intended to reduce the incidences of severe cases of COVID-19 in hospitalized patients. This small target market will further shrink as rapid testing, vaccines, and pre-hospital treatments become available.
By Eduardo Longoria
References
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









