Patent Landscape Analysis of Gene Therapy
In recent years, gene therapy has become a popular product in the biopharmaceutical industry, attracting investment from many companies and resulting in numerous large mergers and acquisitions. With continuous breakthroughs in academic research and increasingly positive clinical trial results, gene therapy is one of the most promising therapies today. As long as drug development, product lines, and commercialization technology are integrated, the potential of gene therapy technology is expected in the future.
Gene therapy patents can be classified according to carrier type and use, including reverse transcriptase virus carriers, lentivirus carriers, adenovirus carriers, and non-virus carriers such as cationic polymers, liposomes, and nanoparticles; the use part includes cancer, Parkinson’s disease, Huntington’s disease, retinal degeneration, muscular dystrophy, Thalassemia and sickle cell disease.
In this article, we retrieve global patents related to gene therapy technology and briefly analyze the areas of delivery carriers, CRISPR/Cas systems, and their uses. Currently, there are 145,933 global patents related to gene therapy, with adeno-associated virus (AAV) carriers being one of the most common virus carriers. No matter in preclinical or clinical development stages, AAV carriers have been widely used, and patents related to AAV carriers account for 8,701 out of the patents related to gene therapy technology.
Related Article: Patent Landscape Analysis of Canine Vaccines
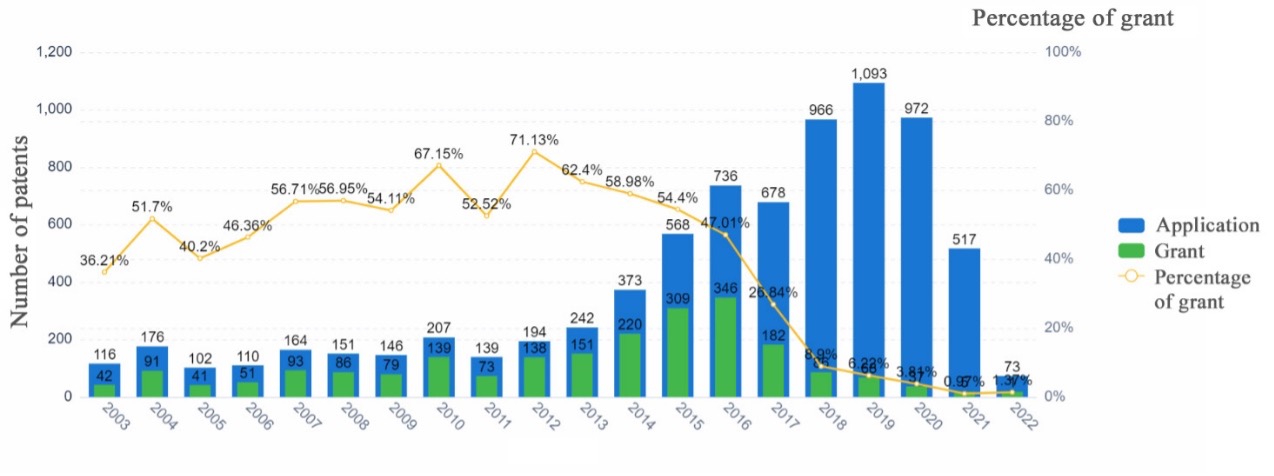
Trend of the patent application and grant
Take the AAV carrier in gene therapy technology as an example. In 2012 the European Medicines Agency (EMA) approved the world’s first AAV gene therapy, Glybera, to treat lipoprotein lipase deficiency patients. Since then, research has progressed rapidly, and the patent application has increased dramatically. Despite this, the number of granted patents has not kept pace with the rapid growth in patent applications.
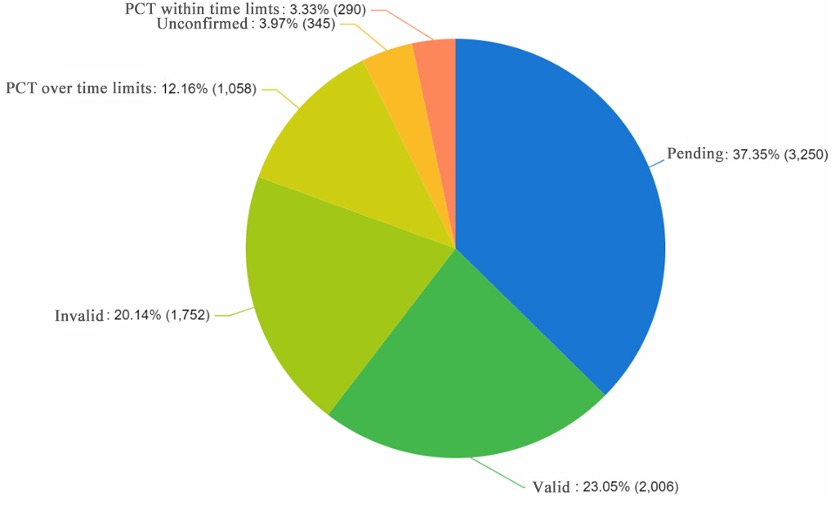
Legal status
In the related patents for gene therapy AAV carriers, the proportion of patents that remain valid is 23.05%, the proportion of patents that have become invalid is 20.14%, and the proportion of patents under review is as high as 37.35%, which indicates that the related technology is in a vibrant stage of development.
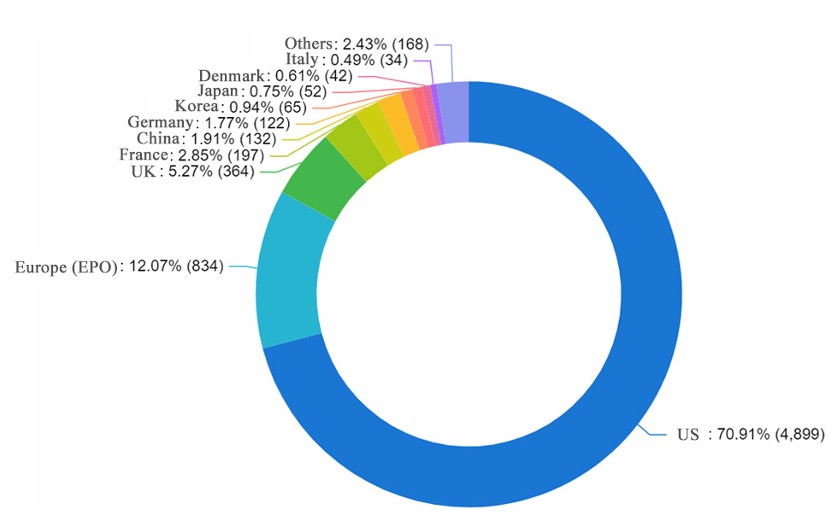
Ranking of countries/regions for technological expertise
In the first approved gene therapy, French Anderson and Michael Blaese of the National Cancer Institute (NCI) successfully delivered a healthy ADA gene to a patient with severe combined immunodeficiency (SCID) through a retrovirus. As a result of this success, clinical trials for gene therapy have exploded. The chart below shows that the innovation of AAV carrier gene therapy technology mainly comes from the United States.
Ranking Analysis of patent applicants
In the gene therapy AAV vector-related technology, the top two applicants for the total number of patents are Genzyme Corp. of the United States and Trustees of The Univ of Pennsylvania of the United States.
|
Applicant |
Number of patents |
|
Genzyme Corp. |
492 |
|
Trustees of the Univ of Pennsylvania |
435 |
|
University of Florida Research Foundation, Inc. |
276 |
|
Institut National de la Sante et de la Recherche Medicale Inserm |
227 |
|
Research Institute at Nationwide Children’s Hospital |
218 |
|
Genethon |
212 |
|
The Regents of The University of California |
186 |
|
The Broad Institute Inc. |
176 |
|
The Children’s Hospital of Philadelphia |
176 |
|
UNIQURE IP BV |
174 |
Written by Sherry Wu/CIPIC
Review & Edit by GeneOnline








