Potential Repurposed Drugs Currently in Evaluation for COVID-19
By Pavel Ryzhov, Ph.D.
As people across the world grapple with the rapid spread of coronavirus pandemic, many find themselves wondering what the available treatment options are, how safe and effective are they, and how can they access them? To unpack this complex topic, a few caveats and differentiation need to be addressed first.
Clinical Course and Standard of Care for COVID-19 Patients
According to the Centers for Disease Control and Prevention (CDC), people infected with SARS-CoV-2 may exhibit no symptoms of COVID-19 (asymptomatic), mild symptoms (cough, shortness of breath, fever), moderate and severe symptoms and ensuing complications (lower oxygen levels, pneumonia, and others), requiring hospitalization and varying levels of medical intervention, including supplemental oxygen, or in most severe cases, mechanical ventilation 1.
The current standard of care may vary depending on the symptoms exhibited, the course of disease progression days since testing positive, and several other factors beyond the individuals’ control. It is also worth mentioning that there are no FDA approved drugs to treat COVID-19 or associated symptoms, though a large number of novel and repurposed therapeutics are being currently tested as part of hundreds of clinical trials around the world.
Below is a list of some select repurposed therapeutics that are currently undergoing clinical trials (in phase II and or phase III as of end-April 2020). We have segregated them based on the form of treatment: treatment of the symptoms and inhibiting the growth of the virus (antiviral drugs).
Virus Inhibition
Remdesivir

Gilead’s remdesivir is a broad-spectrum anti-viral drug that was originally developed to combat Ebola virus outbreak 1. When this monophosphoramidate prodrug, analogous to adenosine is metabolized, it is able to inhibit RNA-dependent RNA polymerase (RdRp) activity by incorporating into the newly synthesized RNA strand, halting further replication. While it did not demonstrate similar efficacy as other Ebola treatments, its human safety profile was nonetheless validated. In addition, Remdesivir was found to inhibit SARS-CoV, MERS-CoV, and bat CoV strains 2 and more recently, showed clinical improvement in COVID-19 patients in an open-label study under a compassionate use program 3.
There are currently 8 registered clinical trials involving remdesivir 5. The first in the US to study the clinical benefits of Gilead’s drug is randomized, controlled trial, comparing remdesivir to placebo is conducted by the National Institute of Allergy and Infectious Diseases (NIAID) 6. Two other main trials aim to evaluate its efficacy as compared to standard of care in patients with severe 7 and moderate COVID-19 8.
The first results from the NIAID clinical trial have just come out 9, announcing that the primary endpoint (time to recovery) was 31% faster in patients administered with remdesivir as compared to placebo (p <0.001), and suggesting a survival benefit (8.0% for the group with remdesivir vs. 11.6% for the placebo group, p = 0.059). In addition, Gilead has just put forth a press release for the preliminary results from another trial for patients with severe COVID-19 symptoms 10. There, a 10-day treatment regimen with remdesivir provided similar clinical benefit as a 5-day treatment. In Gilead’s interpretation, the 5-day regimen has clinical potential for some patients, thus, allowing to expand the drug’s availability for more people.
The second clinical trial (for patients with moderate COVID-19 symptoms) is expected to conclude at the end of May 2020 and provide additional insights about the clinical benefits of remdesivir. Other clinical trials include 3 expanded access (compassionate use) trials and 2 trials where remdesivir is compared to SoC and/or other potential therapeutic agents. All of these clinical trials are for patients who have tested positive for COVID-19 and have been hospitalized with moderate to severe symptoms.
Meanwhile, another trial data from China 4 was published recently with a conflicting finding. The authors of the placebo-controlled study, claim that “remdesivir use was not associated with a difference in time to clinical improvement”. They do observe that patients treated with the drug had a numerically faster time to clinical improvement as compared to placebo, but it was not statistically significant.
- Warren TK, et al. (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531(7594):381–385.
- Agostini ML, et al. (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 9(2):1–15.
- Grein J, et al. (2020) Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med:1–10.
- Wang, et al. (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet DOI:https://doi.org/10.1016/S0140-6736(20)31022-9
- https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=remdesivir
- https://clinicaltrials.gov/ct2/show/NCT04280705
- https://clinicaltrials.gov/ct2/show/NCT04292899
- https://clinicaltrials.gov/ct2/show/NCT04292730
- https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
- https://www.gilead.com/news-and-press/press-room/press-releases/2020/4/gilead-announces-results-from-phase-3-trial-of-investigational-antiviral-remdesivir-in-patients-with-severe-covid-19
Hydroxychloroquine & Chloroquine
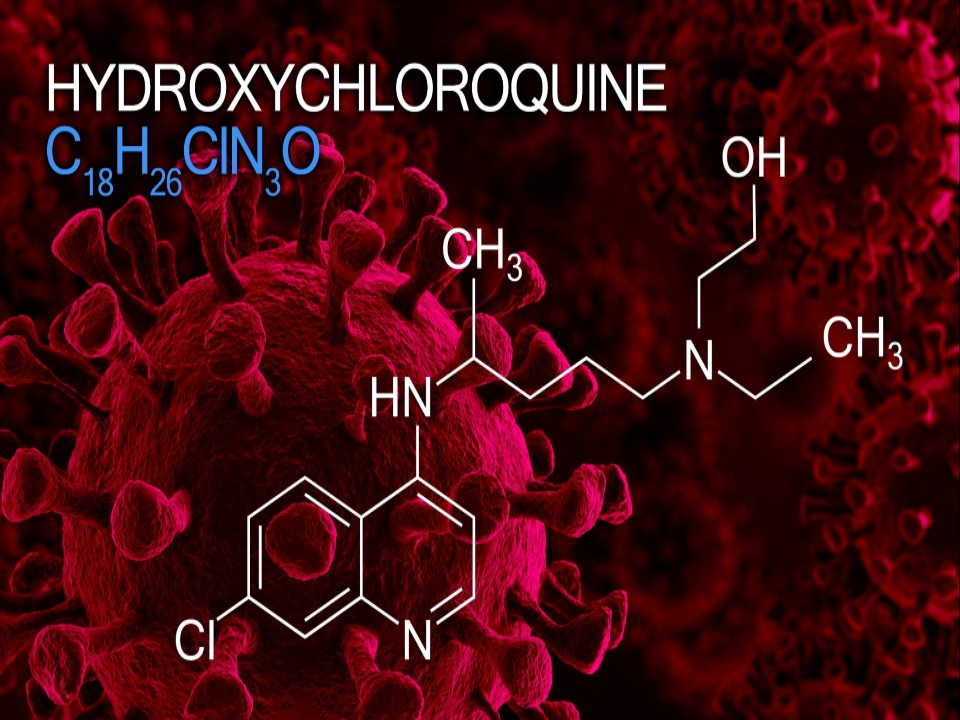
Hydroxychloroquine and chloroquine are widely known small molecule drugs that have been found to show efficacy in treating malaria and autoimmune diseases such as rheumatoid arthritis and lupus 1. Both HCQ and CQ are 4-aminoquinolines and, as weak bases, are able to interact and accumulate in intracellular compartments such as acidic lysosomes, preventing MHC class II-mediated autoantigen presentation, or endosomes, affecting TLR signaling and production of pro-inflammatory cytokines. The exact molecular mechanism of action for HCQ and CQ, however, is tissue and disease dependent and, in the case of COVID-19 have not yet been elucidated 2.
There are now over 130 clinical trials announced or currently recruiting that aim to test the efficacy of HCQ and or CQ in COVID-19 patients 3. Interestingly, another drug that is commonly tested together with HCQ is antibiotic azithromycin. This combination has garnered much media attention after multiple mentions by the White House. However, recent NIH treatment guidelines do not support the treatment of patients with this combination outside of a clinical trial due to potential toxicities 4.
- Schrezenmeier, E, et al. (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nature Rev Rheum 16:155-166
- Fox, R, et al. (1993) Mechanism of action of hydroxychloroquine as an antirheumatic drug. Sem Arthr Rheum 23(2):82-91
- https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=hydroxychloroquine
- https://covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/
Avigan (favipiravir)
Favipiravir is an antiviral drug manufactured by Toyama Chemical (Fujifilm) approved for treating novel or severe influenza in Japan since 2014. It has also gained attention as a treatment for the Ebola virus. As a pyrazinecarboxamide derivative, its mechanism of action is to inhibit RNA-dependent RNA polymerase activity, after it gets phosphorylated intracellularly 1. Due to the similarities in the catalytic domain of RdRp enzyme among various RNA viruses, this antiviral drug has been touted to be efficacious in treating COVID-19, also caused by RNA-based coronavirus.
As of the end of April 2020, there are over 10 registered clinical trials aimed to study the effect of favipiravir on the disease progression among hospitalized patients with mild to moderate symptoms with earliest results to be available in July 2020 2.
Related Article: Japan Trials Asthma Drug Alvesco to fight COVID-19
- Furuta, Y, et al. (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 93(7):449-463
- https://clinicaltrials.gov/ct2/show/NCT04336904?term=favipiravir&cond=COVID-19
Treating the Symptoms
In the context of COVID-19, cytokine storm and resulting lung inflammation have been previously reported and described as one of the underlying causes of disease severity, specifically acute respiratory distress syndrome. Interleukin-6-receptor has been proposed to be a promising inhibitory target because of its ability to bind interleukin-6, a major cytokine, responsible for stimulation of acute phase responses and immune reactions 1.
- Guo, W, et al. (2020) Diabetes is a risk factor for progression and prognosis of COVID-19. Diabetes Metab Res Rev. e3319
Actemra (tocilizumab)
 Actemra (Image Courtesy – Roche)
Actemra (Image Courtesy – Roche)
Tocilizumab is an immunosuppressor drug developed by Roche to treat rheumatoid arthritis (RA) and systemic juvenile idiopathic arthritis and sold under the trade name Actemra after it was approved by FDA in 2010 for these indications 1. As the name suggests, it is a humanized monoclonal antibody developed to bind IL-6R, thus blocking IL-6 cytokine from propagating an immune response in autoimmune diseases, like RA 2.
There are over 20 registered clinical trials where tocilizumab is investigated for its efficacy in the context of COVID-19 infection among hospitalized patients with moderate to severe symptoms 3. Among the major questions that the investigators and clinicians aim to answer is whether early administration of tocilizumab would reduce the chances of requiring mechanical ventilation.
Related Article: Genentech’s Actemra gets FDA approval to begin COVID-19 Trials in April
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125276s114lbl.pdf
- Tanaka, T, et al. (2014) IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb Perspect Biol. 6(10): a016295
- https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=tocilizumab
Kevzara (sarilumab)
Sarilumab or Kevzara is another monoclonal antibody that can bind to IL-6R. It was originally developed by Sanofi and Regeneron Pharmaceuticals to treat RA and approved by FDA in 2017 1. On the heels of the recent preliminary reports from China that tocilizumab has shown efficacy in treating inflammatory response in patients with COVID-19, both companies announced in March that Kevzara’s efficacy will be evaluated in patients with the severe form of COVID-19 2.
There are 10 clinical trials where sarilumab is being compared either to the standard of care, other anti-inflammatory drugs (tocilizumab), anti-virals, or corticosteroids 3. One of the most comprehensive trials is being sponsored by Regeneron and conducted across 63 study locations in the US 4. It aims to study two doses of sarilumab across many patient groups.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf
- http://www.news.sanofi.us/2020-03-16-Sanofi-and-Regeneron-begin-global-Kevzara-R-sarilumab-clinical-trial-program-in-patients-with-severe-COVID-19
- https://clinicaltrials.gov/ct2/results?term=sarilumab&cond=COVID-19
- https://clinicaltrials.gov/ct2/show/NCT04315298
Avastin (bevacizumab)
 Avastin (Image Courtesy – Genentech)
Avastin (Image Courtesy – Genentech)
Bevacizumab (Avastin) is another monoclonal antibody from Roche that was originally approved by the FDA for suppression of blood vessel growth in various cancers (2004) 1. It inhibits vascular endothelial growth factor A (VEGF-A) and based on the previous evidence, the levels of VEGF-A among COVID-19 patients are elevated and may contribute to ARDS because of the increase in vascular permeability and pulmonary edema 2. Currently, 3 clinical trials are underway to investigate whether suppressing VEGF-A will show improved clinical outcomes for COVID-19 patients 3.
Jakafi (ruxolitinib)

Jakafi (Image Courtesy: Business Wire)
Ruxolitinib is a small molecule kinase inhibitor produced by Incyte and approved by the FDA for the treatment of cancers such as myelofibrosis and polycythemia vera 1. It inhibits the JAK1 and JAK2, mediating the cytokine response and immune function. In COVID-19 patients, it is thought to decrease immune hyperactivation and is currently involved in over 10 announced or recruiting clinical trials 2.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









