RemeGen Biosciences to Present New Data Updates in ACR 2022
RemeGen Co., Ltd., a leading biopharmaceutical company with fully integrated capabilities in research and discovery, clinical development, manufacturing, and commercialization of biologic drugs, will join the event of the American College of Rheumatology (ACR) 2022, hosted from November 10th to 14th. This event will present Telitacicept phase III clinical trial data on Systematic lupus erythematosus (SLE) and data from the phase II clinical trial for Sjorgren’s syndrome.
About Telitacicept
Telitacicept is a novel fusion protein composed of a transmembrane activator and CAML interactor (TACI) and the Fc portion of IgG, which targets and neutralizes B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL). BLyS and APRIL are both involved in the development of B cells from pre-B lymphocytes to mature B cells and ultimately to plasma cells, the professional cells producing antibodies, as well as in the co-stimulation of T-cell proliferation under certain conditions. Telitacicept blocks BLyS and APRIL from binding to BAFF-R, BCMA, and TACI receptors expressed on the B-cell surface, suppressing the BLyS and APRIL signaling, and inhibiting the development and survival of mature B cells and plasma cells.
Systemic lupus erythematosus (SLE) is the principal indication of Telitacicept. Through priority review, Telitacicept was approved by China National Medical Products Administration (NMPA) and is included in the National Reimbursement Drug List (NRDL). Telitacicept also received FDA Fast Track Designation as a treatment for SLE with an ongoing global Phase III study.
In addition to SLE, the company is actively developing Telitacicept for six other B cell-mediated autoimmune diseases and refractory autoimmune diseases with unmet clinical needs in China, including Neuromyelitis optica spectrum disease, rheumatoid arthritis, IgA nephropathy, primary Sjogren’s syndrome, relapsing-remitting multiple sclerosis, and systemic myasthenia gravis.
Telitacicept for Systematic Lupus Erythematosus, data from phase III trial
This is a 52-week, randomized, double-blind, placebo-controlled, phase III clinical trial (NCT04082416). SLE patients aged 18 to 65 years with positive ANA and/or anti-dsDNA and a SELENA-SLEDAI score ≥8 were randomized 1:1 to receive either Telitacicept 160 mg or placebo subcutaneously once weekly in combination with standard therapy for 52 weeks.
A total of 335 patients were randomized to receive Telitacicept 160 mg (n=167) or placebo (n=168). Of the randomized patients, 236 (70.4%) completed 52 weeks of treatment. The primary endpoint at week 52 revealed a significant proportion of patients in the Telitacicept 160 mg group achieving SRI4 response compared with the placebo group (82.6% vs. 38.1%, p< 0.001). The SRI4 response was significantly greater in the Telitacicept group compared with the placebo group as early as Week 4, and the differences sustained between the 2 groups until week 52 (p < 0.01 for all time points) (Fig.1).
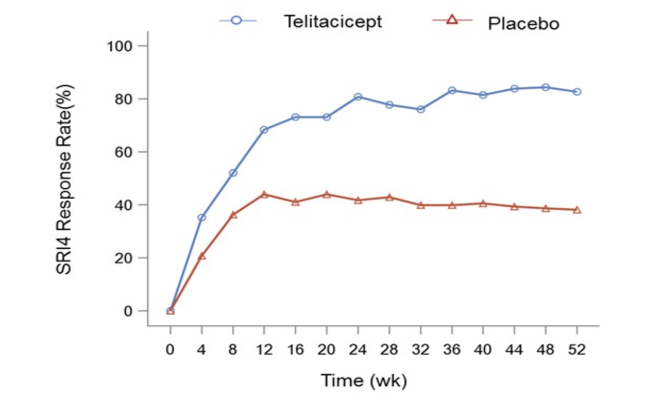
Regarding safety, 91.6% of subjects in the Telitacicept group and 84.5% in the placebo group had treatment-emergent adverse events (TEAEs). There were 17 serious adverse events (SAEs) in 12 subjects (7.2%) treated with Telitacicept and 36 SAEs in 24 subjects (14.3%) treated with placebo. The most common AEs (≥10%) with Telitacicept were upper respiratory tract infection, decreased IgG and IgM, injection site reactions, and urinary tract infection. No death occurred during the study.
Telitacicept for Sjogren’s Syndrome, data from phase II trial
The upcoming event will also present data to evaluate the efficacy and safety of Telitacicept in adult patients with primary Sjogren’s syndrome (pSS) in phase II randomized, double-blind, placebo-controlled trial.
A total of 42 patients with pSS with positive anti-SSA antibody and ESSDAI≥ 5 were randomly assigned, in a 1:1:1 ratio, to receive weekly subcutaneous Telitacicept 240 mg, 160 mg, or placebo for 24 weeks. Thirty patients who completed their 24 weeks visit were included in the per-protocol set (PPS) (Telitacicept 240 mg, n=8; Telitacicept, 160 mg; n=12; placebo, n=10).
The primary endpoint was the change from baseline in the ESSDAI at week 24. Results from the trial revealed that Telitacicept 160 mg showed a significant reduction in ESSDAI score from baseline to week 12 and week 24 compared with placebo (p< 0.05); Telitacicept 240 mg resulted in a considerable decrease in ESSDAI score from baseline to week 24 compared with placebo in the PPS population (p< 0.05). For the secondary endpoint, Telitacicept can significantly reduce from baseline in MFI-20 in weeks 12 and 24. No serious adverse events were observed in the Telitacicept treating group. (Fig.2).
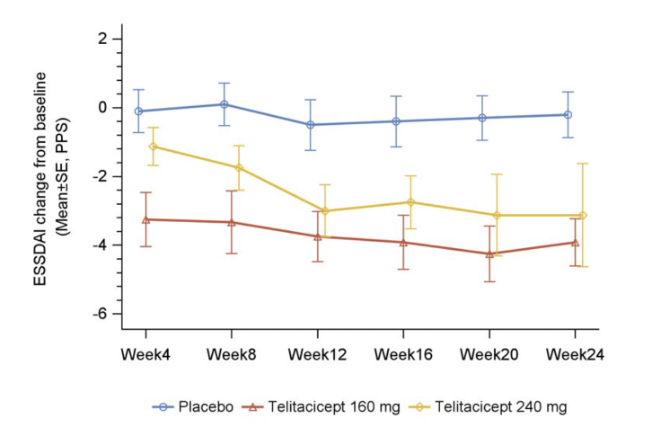
About RemeGen
RemeGen, Ltd. (“RemeGen”) is a leading biopharmaceutical company in China dedicated to fulfilling unmet medical needs for patients with life-threatening conditions. RemeGen’s main focus is research and development, manufacturing and commercialization of novel biologics, most notably monoclonal antibodies (mAb) and antibody-drug conjugates (ADCs). Since its inception in 2008, RemeGen has created more than 10 novel drug molecules in various clinical development stages. Currently, there are two products in late-stage clinical development to treat autoimmune and oncology indications. Headquartered in Yantai, Shandong Province, China, RemeGen has labs/offices in Beijing and California.
For more information about RemeGen, please visit: www.remegen.com
For those attending ACR, please come and visit us at our booth #2357
Investor Contact
Annaly Godwin
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com








