RNA Therapeutics: An Overview
For ages, the biopharmaceutical market has been dominated by small molecules that predominantly targeted cellular proteins. And yet, a majority of them have been undruggable. In the past couple of decades, groundbreaking research in the discipline of RNA biology has led to the development of a new class of biologics that constitute RNA therapeutics. Although the inherent properties of RNA molecules throw a few challenges during drug development, it still holds certain advantages over existing therapies. Here, we review the recent growth in the field and the promise of some newly developed modalities.
Pharmacological therapies have played a big part in our fight against several life-threatening diseases. Despite that, several illnesses exist for which drug candidates have either not been identified yet or the ones that have, failed to offer a complete cure. Even with an abundance of information with regard to disease-causing determinants, a lot of targets have remained “undruggable”. The majority of the FDA-approved drugs in the market have been small molecule inhibitors that act on proteins such as enzymes, hormones or receptors. Yet, that encompasses only a small population of cellular proteins. Protein-based therapies such as antibodies exhibit better binding specificity to targets but pose other difficulties with respect to their size and stability. This is further compounded by the problem of DNA variants and acquired drug resistance. Therefore, there is always a growing need for pharmacological interventions with newer biologics.
The Strengths and Weaknesses of RNA Therapeutics
The advances in the RNA biology field with seminal discoveries such as RNA interference and the identification of diverse non-coding RNAs have furthered the integration of this macromolecule as a key component in developing therapies. Although various modalities of RNA therapies have been investigated in the past twenty years, only 10 drugs have been approved by the FDA so far (Figure 1).
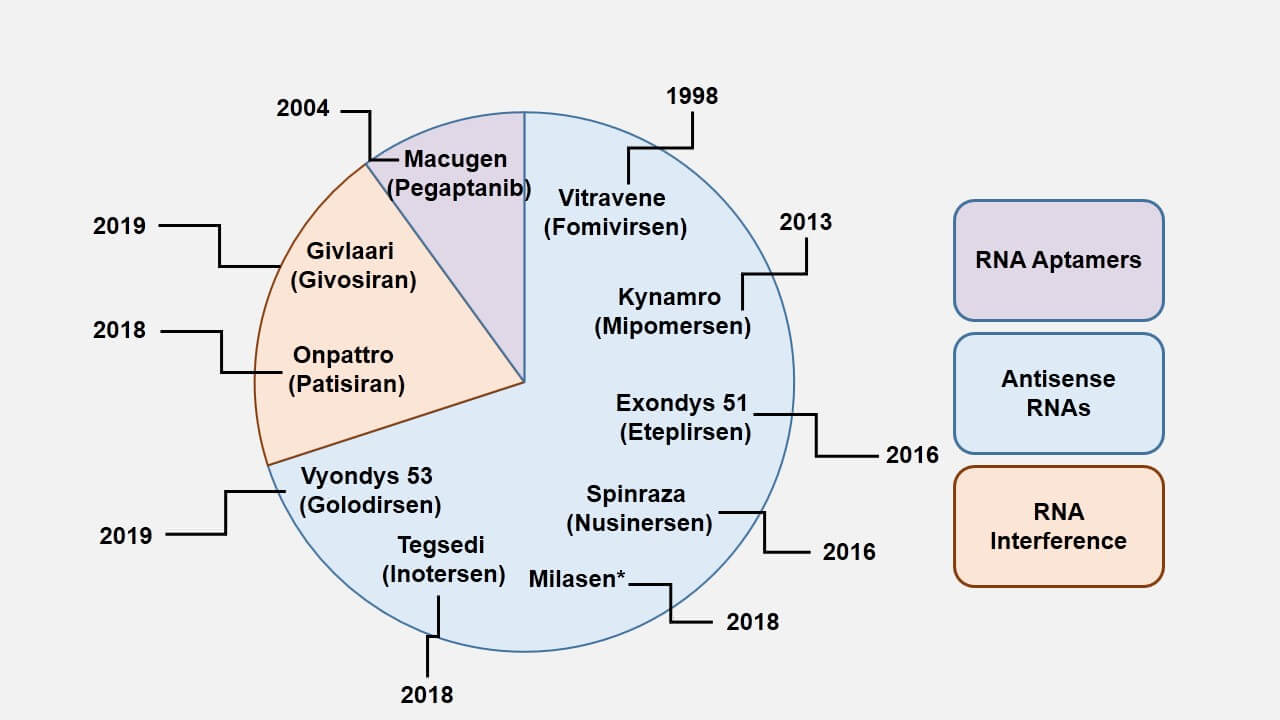
Figure 1: FDA-approved RNA therapies over the years. *Milasen is a special case that received approval with an n of 1 patient.
The major advantage of some RNA therapeutics over small molecules is that it binds to the mRNA and leads to its degradation, thereby preventing a disease-causing protein from being translated. Another advantage is the ease with which it could be integrated with existing therapies. However, their unstable nature and propensity to get degraded by ribonucleases is a major impediment, along with their immunogenic properties. Another arduous task is to find the right target cell and optimize an appropriate strategy to deliver the drug past its lipid bilayer. Once, this is figured out, in principle, it is relatively easier to develop the therapeutic as compared to traditional drugs.
The monstrous growth of CRISPR/Cas9 technology in recent times has taken RNA-based therapeutics to the next level where gene expression is altered by inducing chromosomal mutations. It is only beginning to influence RNA editing and covering that field is beyond the scope of the article. Let’s take a look at some of the other classes of RNA therapies that have been under development for the past couple of decades (Figure 2).
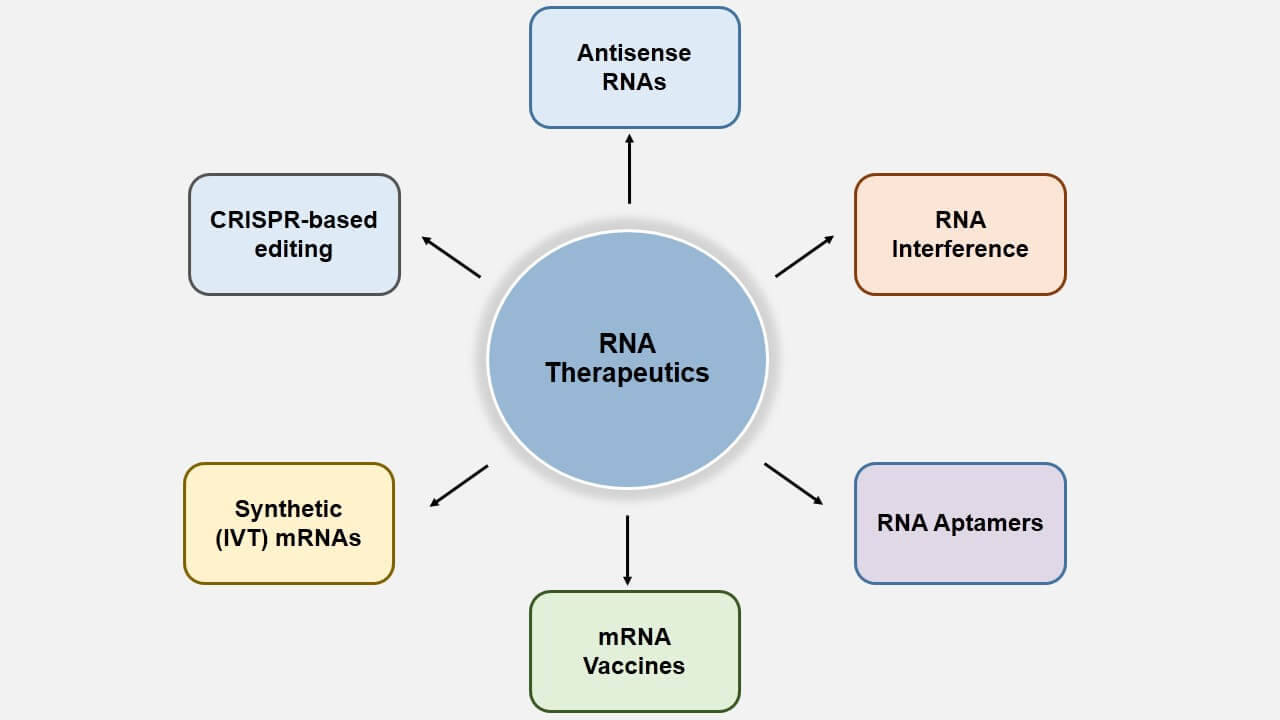 Figure 2: Different classes of RNA Therapeutics currently available
Figure 2: Different classes of RNA Therapeutics currently available
Antisense RNA Drugs
Antisense oligonucleotide (ASO) based drugs follow the simple principle of complementary base pairing with target mRNAs and is, therefore, an attractive prospect for therapies. The seed for this idea was sowed way back in 1978 when a specific oligodeoxyribonucleotide was designed to inhibit the RNA translation of Rous sarcoma virus. In order to deal with hindrances such as ribonucleases, ASOs are subjected to a number of chemical modifications using newly invented medicinal chemistry. Modifications in base, sugar, internucleoside linkage or usage of small and large molecule conjugates have all significantly improved their pharmacokinetics, stability, binding affinity, and potency. Some common modifications include 2’-O-methyl, 2’-Fluoro, 5-methylcytosine and 2’-O-methoxyethyl substitutions.
Vitravene (Fomivirsen) was the first ASO to be approved by the FDA in 1998 for the treatment of cytomegalovirus retinitis (CMV) in immunocompromised patients. Although the marketing of the drug was stopped in a few years following advances in antiretroviral therapy, it inspired a slew of other drugs belonging to this class. Likewise, Kynamro (Mipomersen) was approved for the treatment of homozygous familial hypercholesterolemia but it resulted in severe adverse effects including liver damage.
Ionis Pharmaceuticals’ Domination in Recent FDA Approvals
In 2016, two ASOs, Exondys 51 and Spinraza, garnered FDA approvals and became the first drugs to get market authorizations for their respective disease indications. Sarepta Therapeutics’ Exondys 51 (Eteplirsen), received accelerated approval to treat Duchenne muscular dystrophy (DMD), caused due to a confirmed mutation in the dystrophin gene. This antisense oligonucleotide contains a phosphorodiamidate morpholino modification and it functions by triggering the excision of exon 51 during pre-mRNA splicing, thus correcting the downstream reading frame of dystrophin.
Similarly, Spinraza (Nusinersen) also alters RNA splicing and was developed for the treatment of Spinal Muscular Atrophy (SMA), caused due to a homozygous loss or mutation of the survival motor neuron 1 (SMN1) gene. It has a couple of modifications, a 2’-O-methoxyethyl substitution and a phosphorothioate (PS) linkage which reduces the hydrophilicity, increases stability and enhances protein binding, all of which are critical for cellular uptake and intracellular distribution. The drug target, which was originally discovered by Dr. Ravindra Singh’s team from UMass Medical School, was licensed to Ionis Pharmaceuticals (formerly Isis Pharmaceuticals). It went on to develop a drug compound in collaboration with Prof. Adrian Krainer from Cold Spring Harbor Labs. A commercial partnership with Biogen eventually led to the latter acquiring exclusive rights for the development and commercialization of Spinraza.
Ionis Pharmaceutical’s, Tegsedi (Inotersen) received FDA approval last year to treat polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The ASO has a number of modifications including 2’-O-methoxyethyl, 5-methyluridine,5-methylcytosine, sodium salt, and PS linkages. Last year, Waylivra (Volanesorsen), another ASO of Ionis and it’s subsidiary Akcea Therapeutics suffered rejection at the hands of the FDA on the basis of safety. However, in May 2019, it received EU approval for the treatment of adult patients with familial chylomicronemia syndrome (FCS). Significant clinical trial data and EU approval have encouraged the companies to try again for authorization in the US. As of today, Ionis has a market cap of $8.70 billion and its diverse pipeline boasts of many best-in-class medicines in various stages of clinical trials, to treat a broad range of diseases including cancer, cardiovascular, neurological, infectious and pulmonary diseases.
In December 2019, Sarepta Therapeutics received conditional approval from the FDA for yet another treatment for DMD. Vyondys 53 (Golodirsen) was initially rejected but surprisingly received FDA authorization following a formal dispute resolution request and resubmission of the NDA.
ASOs for Personalized Medicine
Last year witnessed a remarkable case where an ASO, Milasen was quickly manufactured and FDA approved to save a single patient, making it a pioneering example of personalized medicine. Taking a leaf out of Spinraza’s book, in 2017, Dr. Timothy Yu and his colleagues from Boston Children’s Hospital designed an oligonucleotide to treat a rare and fatal neurodegenerative condition called Batten disease in a 6-year-old patient, Mila Makovec. What followed was a race to deliver the drug for Mila, which was accomplished by setting a record pace in drug development.
RNA Interference Drugs
RNA interference is a cellular mechanism through which small, non-coding RNAs such as siRNAs and miRNAs control gene expression through transcriptional or post-transcriptional gene silencing. While endogenous miRNAs recognize and bind to target mRNAs even with limited complementary base pairing leading to its cleavage or translational repression, siRNAs are made from exogenous dsRNA precursors and binds with perfect complementarity. Both classes of RNAs require the host’s endogenous machinery for their production.
The discovery of this phenomenon in nematodes was a watershed moment in RNA biology and it went on to impact various other fields with its useful tools to alter gene expression. Concurrently, the interest of drug developers on RNAi increased and many siRNA therapeutics reached clinical trials. As with the case of ASOs, RNAi drugs are also subjected to modifications to enhance potency and elude nonspecific binding. Alnylam Therapeutics is one of the well-known RNAi therapeutics company founded in 2002 with a current market cap of $13.12 billion. Its Onpattro (Patisiran) became the first RNAi drug in history to be approved by the FDA. Patisiran is currently marketed for the treatment of hereditary transthyretin amyloidosis (hATTR). On November 20th, the company announced the FDA approval of Givlaari (Givosiran), targeting aminolevulinic acid synthase 1 (ALAS1) for the treatment of acute hepatic porphyria (AHP).
Dicerna Therapeutics is a smaller RNAi company but is touted to emulate the successes of Alnylam and Ionis in a few years. It has invented a new technology called GalXCTM with which it advances next-generation RNAi therapies. The GalXC-based therapies are processed by the Dicer enzyme which initiates the RNA interference mechanism to silence disease-causing genes in the liver. The company has a number of candidates in preclinical and clinical trials, but its most advanced therapy is DCR-PHX for Primary Hyperoxaluria, a rare condition where the kidneys cannot function properly to filter waste from the body.
Similarly, a number of miRNA mimic-based treatments are in clinical development or early clinical trials. These drugs mimic the endogenous miRNAs and bind to their targets to induce gene silencing. The loss of endogenous miRNAs is well known to result in the proliferation and progression of tumors. In 2017, a research publication reported on MesomiR-1, the first-in-human Phase I study of microRNA-loaded minicells (EnGeneIC Dream Vectors) called TargomiRs. They are miR-16-based miRNA mimics that targeted EGFR in preclinical models of malignant pleural mesothelioma. It remains to be seen how this novel treatment approach would progress. Miragen, MiRNA Therapeutics (now Synlogic), and Regulus Therapeutics are some companies that are exclusively developing miRNA drugs. Although none of them have reported drugs that progressed to phase 3 trials yet, many are in active phase 2 trials.
RNA Aptamers
Unlike the above-mentioned classes, RNA aptamers interact directly and block a protein product. RNA aptamers are selected through multiple rounds of in vitro or cell-based process called systematic evolution of ligands by exponential enrichment (SELEX). Using this process, a number of unique sequences can be picked from RNA libraries to bind to protein targets. They function similar to that of antibodies but are easier to manufacture and their small size allows interaction with a target protein’s hard to access binding sites. They have relatively better structural stability, highly resistant to heat and like other RNAi therapeutics, it can also be modified by chemicals.
Macugen (Pegaptanib) was the first Aptamer to be FDA approved for the management of neovascular age-related macular degeneration (AMD). It was originally designed for cancer treatment as an anti-VEGF aptamer. Similarly, a number of other aptamers that target EGFR, HER2, HER3, PD-1/PD-L1. CD-40 and nucleolin have been developed and have been shown to demonstrate anti-tumor properties in clinical trials. At the moment, Zimura (avacincaptad pegol), an inhibitor of the Complement C5 is under clinical trials as a combination therapy with Lucentis for the treatment of AMD.
mRNA Therapeutics: Synthetic (IVT) mRNAs and Vaccines
In vitro-transcribed (IVT) synthetic mRNA is an emerging class of RNA therapeutics in which the mRNA molecule introduced into a cell can produce a functional protein. As early as the 1990s, Wolf et al. demonstrated that the host cell’s protein synthesis machinery can translate IVT mRNA into the desired protein. It could be an antibody, a protein antigen, a cytokine, or a gene-editing protein. But the concept gained traction only when modifications improving mRNA stability and translation efficiency were optimized. Just as the case with endogenous mRNAs, the IVT mRNA template must also contain optimized structural elements such as 5’ cap, 5′ UTR, coding sequence, 3′ UTR, and a polyA tail to improve efficacy and prevent unwelcomed immunogenic reactions.
However, their immunogenic nature becomes a favorable feature, when they are developed as mRNA vaccines. Compared to the traditional vaccines, mRNAs offer a much better alternative with their potent and long-lasting qualities. Besides, their rapid manufacturing capabilities and the ability to elicit an immune response similar to a viral infection is a huge advantage. A number of mRNA vaccines are currently in human clinical trials. Moderna Therapeutics, a clinical-stage biotechnology company is pioneering messenger RNA (mRNA) therapeutics and vaccines and has a handful of drugs in its pipeline. In September, it announced a multi-year research collaboration with Harvard University. It also will establish an initiative at Harvard Medical School called the Alliance for RNA Therapies for the Modulation of the Immune System (ARTiMIS), which will enable basic science research in the field of immunology using its mRNA platform and nanoparticle delivery technology.
Major Challenges and the Future of RNAi Therapeutics
Apart from poor pharmacokinetics, stability and non-specific binding, the major drawback of some RNA drugs, especially ASOs and siRNA therapeutics is their poor cellular uptake. RNA drugs must surpass the lipid bilayer and must enter the cell through the process of endocytosis. Once entered, they must achieve endosomal escape to get to the cytoplasm. RNAs must be transfected with cationic lipids or other types of nanoparticles to physically protect the RNAs from getting degraded and aid in cellular uptake and endosomal escape. However, this approach is not completely safe as lipid nanoparticles can cause toxicity in the liver. Another strategy is to conjugate the RNA with a targeting ligand such as N-acetylgalactosamine (GalNAc). GalNAc is an amino sugar that aids in the efficient targeting of RNA into the liver due to the high expression of its receptor on the surface of hepatocytes.
In conclusion, the new field of RNA therapeutics is progressing steadily. Albeit the authorization of only a handful of RNA drugs so far, it gives a big motivation for the rest of them in clinical trials. It is safe to assume that in the coming years RNA therapeutics would feature more frequently in pharmacological endeavors and reduce the count of undruggable protein targets and cure diseases.
Related Article: The Rejuvenated Promise of RNA Therapeutics
References
- https://www.ncbi.nlm.nih.gov/pubmed/29617640
- https://www.ncbi.nlm.nih.gov/pubmed/30521885
- https://www.ncbi.nlm.nih.gov/pubmed/29662218
- https://www.ncbi.nlm.nih.gov/pubmed/31597037
- http://investors.alnylam.com/news-releases/news-release-details/alnylam-announces-first-ever-fda-approval-rnai-therapeutic
- http://investors.alnylam.com/news-releases/news-release-details/alnylam-announces-approval-givlaaritm-givosiran-us-food-and-drug
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5835633/
- https://www.ncbi.nlm.nih.gov/pubmed/1690918
- https://www.ncbi.nlm.nih.gov/pubmed/30795778
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6453554/









