TALED Tool Launches a New Era of Mitochondrial Genome Editing
Researchers from the Korean Institute for Basic Science developed a new gene-editing platform – TALED (transcription activator-like effector-linked deaminases). TALEDs are DNA base editors capable of performing A-to-G base conversion on the mitochondrial genome. This discovery can be regarded as the final piece of the gene-editing technology puzzle.
CRISPR Opened Up New Doors for Treatments
From the identification of the very first restriction enzyme in 1968 to the invention of polymerase chain reaction (PCR) in 1985 and the demonstration of CRISPR-mediated genome editing in 2013, each breakthrough discovery in biotechnology improved our power to manipulate the blueprint of life – DNA. Particularly, the CRISPR-Cas system’s development opened up new possibilities for treating previously incurable genetic diseases.
Severe Diseases Due to Mitochondrial DNA Mutations
Known as the “powerhouse of the cell”, mitochondria are cellular organelles that serve as energy-generating factories. As it is an important organelle for energy metabolism, if a gene is mutated, it causes an array of genetic diseases related to energy production.
Director Kim Jin-Soo of the Center for Genome Engineering said, “There are some extremely nasty hereditary diseases arising due to defects in mitochondrial DNA (mtDNA). For example, Leber hereditary optic neuropathy (LHON), which causes sudden blindness in both eyes, is caused by a single point mutation in mitochondrial DNA.” Currently, there are no effective treatment options for the disease, with undetermined global prevalence. 1/30,000 to 1/50,000 in Northern England and Finland are found with the condition.
Another mitochondrial gene-related disease includes mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS). The rare disorder begins in childhood, usually between 2 and 15 years of age, and mostly affects the nervous system and muscles. The most common early symptoms are seizures, recurrent headaches, stroke-like episodes, and this can lead to altered consciousness, vision, hearing loss, loss of motor skills, and intellectual disability.
Mitochondrial DNA Editing’s Evolution
The mitochondrial genome is inherited maternally, there are 90 identified disease-causing point mutation sites in mitochondrial DNA, affecting at least 1 in 5,000 individuals.
Existing genome editing tools are helpless due to limitations in their delivery method to the mitochondria. For example, the CRISPR-Cas platform is inapplicable for fixing mitochondrial gene mutations, because the guide RNA cannot enter the organelle itself. “Another problem is that there is a dearth of animal models of these mitochondrial diseases. This is because it is currently not possible to engineer mitochondrial mutations necessary to create animal models,” Director Kim added. “The lack of animal models makes it very difficult to develop and test therapeutics for these diseases.”
In 2020, a new base editor named DddA-derived cytosine base editors (DdCBEs) that can perform C-to-T conversion from mitochondrial DNA was created by Harvard Broad Institute and MIT researchers led by David R. Liu. It was made possible by a new technology called base editing, which converts a single nucleotide base into another without messing with the DNA. However, this technique also had its limitations. Not only is it restricted to C-to-T conversion, but it is mostly limited to the TC motif, making it effectively a TC-TT converter. This means that it can correct only 9 out of 90 (10%) confirmed pathogenic mitochondrial point mutations.
TALED’s Ability to Perform A-to-G Conversion
First author Cho Sung-Ik shared, “We began to think of ways to overcome these limitations. As a result, we were able to create a novel gene-editing platform called TALED that can achieve A-to-G conversion. Our new base editor dramatically expanded the scope of mitochondrial genome editing. This can make a big contribution not only to making a disease model but also to developing a treatment.” Also, note that 39 out of the 90 known disease-causing mutations could be corrected by performing A-to-G conversions in human mtDNA.
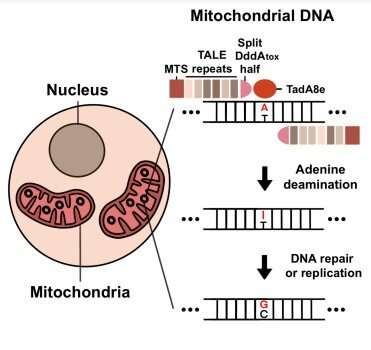
The researchers combined three components to create TALED, The first component is a transcription activator-like effector (TALE), capable of targeting a DNA sequence. The second component is TadA8e, an adenine deaminase for promoting A-to-G conversion. The third component, DddAtox, is a cytosine deaminase that makes DNA more accessible to TadA8e.
A fascinating aspect of TALED is TadA8e’s ability to perform A-to-G double-stranded DNA (dsDNA) editing in mitochondria. Director Kim said, “No one has thought of using TadA8e to perform base editing in mitochondria before, since it is supposed to be specific to only single-stranded DNA.” It was this thinking outside of the box approach that has helped them invent TALED.
Researchers hypothesized that DddAtox improves accessibility to dsDNA by transiently unwinding the double-strand. This brief but temporary time window allows TadA8e, a super fast-acting enzyme, to perform edits quickly. In addition to tweaking TALED components, they also developed a technology that is capable of A-to-G and C-to-T base editing simultaneously, as well as A-to-G base editing only. The group demonstrated this new technology by creating a single cell-derived clone containing desired mtDNA edits. TALEDs were found to be neither cytotoxic nor cause mtDNA instability. Additionally, there was no undesirable off-target editing in nuclear DNA and very few off-target effects in mtDNA. The Korean researchers now aim to further improve the TALEDs by increasing efficiency and specificity, eventually paving the way to correct disease-causing mtDNA mutations in embryos, fetuses, newborns, or adult patients.
Written by Miggy Chang
Translated by Fujie Tham









