The Evolution of Lung Cancer Therapies at a Glance
By Ruchi Jhonsa, Ph.D.
In 1910, esteemed surgeon Alton Ochsner recalled, “As a student, I was asked to witness an autopsy of a patient with lung cancer because I was told that it is so rare that I might not be able to see such a case again. However, 20 years later, I witnessed a sudden surge in the number of lung cancer patients, which coincided with a surge in tobacco sales and consumption.” Today in the United States, the combined fatalities from breast, colon, and prostate cancer together are much lesser and over 150,000 patients died of lung cancer last year (2019). Significant efforts are taken to bring new treatments and progress existing ones to treat and improve the quality of life.
The Early Days
It was in the year 1955 when a sudden surge in lung cancer victims was witnessed. U.S. Surgeon General and the U.K. Royal College of Physicians noticed a clear correlation between rising lung cancer cases and tobacco consumption. However, people did not easily digest this study, so much so that, the first statutory warnings of smoking appeared on cigarette boxes only 20 years later. At the time, surgery was the best therapeutic option available. Depending upon the location of cancer in the lungs, either a lobe of the lung (lobectomy) or two (bilobectomy) or the entire lung (pneumonectomy) was removed.
The entry of chemotherapy in the ensuing years was a significant upgrade. The treatment regimen combining doxorubicin with cyclophosphamide, vincristine and radiation therapy was found to be effective in reducing tumor size in some small cell lung cancers (SCLC). This breakthrough sparked several studies that researched on combination chemotherapy at the time. Amidst all of this, researchers were still asking the fundamental question-what drives lung cancer? The seminal discovery of epidermal growth factor receptor (EGFR) involvement in lung cancer progression paved the path for identifying various driver genes, that formed the basis of several targeted therapies in the early 2000s.
The Augmented Efficacy of Chemotherapy-Radiation Combo
A few years later, an important milestone in the field was achieved when two studies showed the increased efficiency of radiation and chemotherapy combination in treating third stage non-small cell lung cancer (NSCLC). This two-pronged approach soon became the standard of care for this disease. Thereafter, several new chemotherapy drugs emerged and were found to be effective particularly when combined with cisplatin. The list included paclitaxel (Taxol), docetaxel (Taxotere), vinorelbine (Navelbine) and gemcitabine (Gemzar). Chemotherapy’s popularity amongst doctors made it a go-to treatment for lung cancer following radiation therapy, surgery or supportive care. It was shown that following surgery, a round of chemotherapy could extend survival by as much as 5%. However, cancer still relapsed in numerous cases. In 1996, topotecan, a topoisomerase I inhibitor got approval for intravenous use and became the new standard of care for relapsed SCLC in many patients.
Targeted Therapy Outperforms Chemotherapy
By now it was clear that mutations in the EGFR gene are a major contributor to disease progression. Building on this knowledge, the first targeted therapy inhibiting EGFR, gefitinib (Iressa) was developed and FDA approved for NSCLC. Research suggested that the drug was superior to general chemotherapy in patients who were carrying EGFR mutations. Soon, Genentech joined the race and received authorization for their EGFR inhibitor, erlotinib for patients with advanced NSCLC. These targeted therapies were very effective in improving the survival rate. As time progressed, a new variety of treatments were conceptualized and implemented. In 2006, a major study showed that bevacizumab (Avastin) extends survival in combination with standard chemotherapy. The drug worked by blocking VEGF and preventing the formation of new blood vessels, some of which turn out to be tumor feeding. Soon after, it received the FDA nod for treating non-squamous NSCLC.
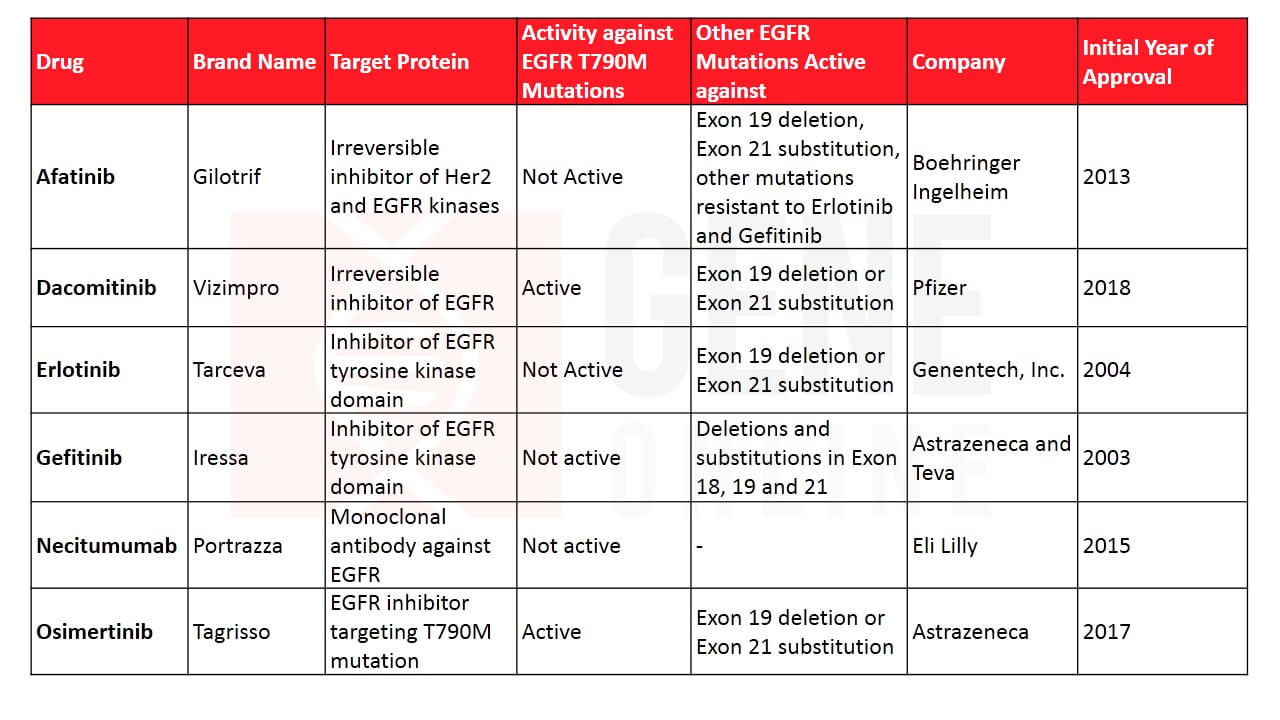 Table 1: Lung cancer therapies targeting EGFR
Table 1: Lung cancer therapies targeting EGFR
Targeted therapies revolutionized the entire field and it became extremely important for its evolution to understand what drives cancer. This resulted in the launch of the modern gene mapping project that aimed to develop a comprehensive atlas of the genetic abnormalities in lung cancer. Besides, EGFR, gene abnormalities were discovered in the anaplastic lymphoma kinase (ALK) pathway, ROS-1, BRAF, NRTK-1, and NRTK-2. ALK mutation was found in only 4% of lung cancers and is more common in patients who never smoked. In 2011, the drug crizotinib (Xalkori) targeting the ALK pathway was granted FDA approval. In 2017, dabrafenib and trametinib got FDA approval for treating NSCLC with BRAF gene alterations and larotrectinib for NRTK-1/NRTK-2 alterations. While inhibitors for most of these altered proteins are now developed, the ones targeting drivers such as RET, HER2, MEK, and MET gene are currently being tested.
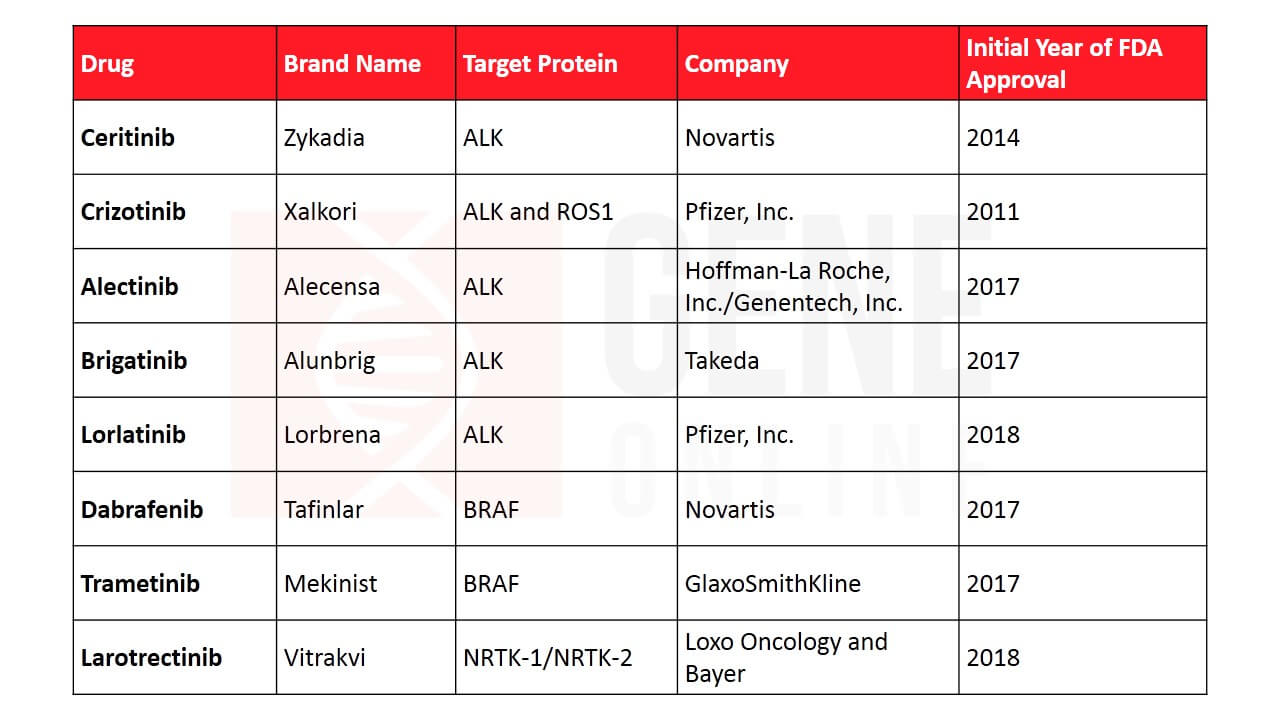 Table 2: Lung cancer therapies targeting other driver genes
Table 2: Lung cancer therapies targeting other driver genes
Immunotherapy-A Primitive Treatment
A primitive version of immunotherapy was tested for cancer more than a century ago, but researchers were puzzled why it failed to work consistently at all times. Through years of research, it was finally understood that cancers are very good at eluding the immune system. They trick the body’s defense by utilizing the same pathway that keeps immune cells from attacking our tissues. This pathway comprises of immune checkpoint proteins PD-1 and PD-L1, which are present on the T-lymphocytes. The modern immunotherapy drugs mask this pathway and heighten the immune response against cancer. The FDA approved three new immunotherapies targeting PD-1 for NSCLC patients who currently or previously were under chemotherapy, namely nivolumab (Opdivo), atezolizumab (Tecentriq), and pembrolizumab (Keytruda) in 2015. Last month, the FDA approved AstraZeneca’s durvalumab for treating extensive-stage SCLC in combination with chemotherapy. This mode of therapy has established a new paradigm for lung cancer treatment and has become a preferred choice over chemotherapy regimens for PD-L1 positive cancers.
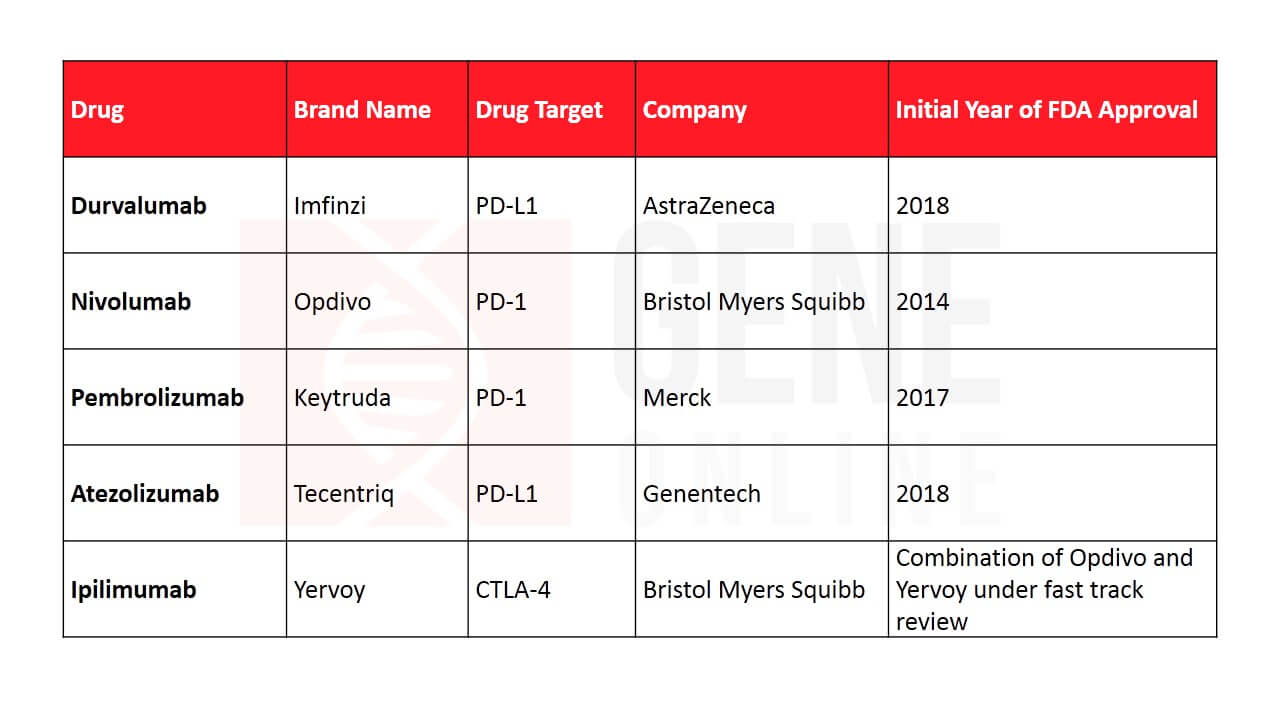 Table 3: FDA approved immunotherapies for lung cancer
Table 3: FDA approved immunotherapies for lung cancer
Another way scientists combat cancers is through decorating the immune cells with receptors specific for tumor antigens. Commonly known as adoptive cell therapy, the approach extracts tumor-reactive lymphocytes from the patient’s blood and modifies it to recognize and target tumor-associated antigen, thereby establishing tumor immunity. Adoptive cell therapy can either make lymphocytes express novel tumor-reactive T cell receptors (TCR) or antibody-based chimeric antigen receptors (CAR T) to antigens on cancer cells. Several clinical trials are ongoing for CAR-T cell therapy but none of them have been approved so far for lung cancers.
The remarkable evidence of cancers diminishing post immune and targeted therapies have led to an explosion of investment from pharmaceutical companies, philanthropists as well as the government. In 150 years, scientists saw lung cancer treatment evolving from general surgery to molecule specific targeted and immunotherapy, increasing in their effectiveness over time. As our knowledge of the immune-cancer interaction grows, more novel and exceptional therapies are anticipated to grace the field.
Editor: Rajaneesh K. Gopinath, Ph.D.
Related Article: TIGIT Immunotherapy: A Ray of Hope for Cancer Treatment?
References
- https://www.dovepress.com/clinical-evaluation-of-dacomitinib-for-the-treatment-of-metastatic-non-peer-reviewed-article-DDDT
- https://www.atsjournals.org/doi/full/10.1164/rccm.200504-531OE
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4607819/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3527990/
- https://www.ncbi.nlm.nih.gov/pubmed/30539506
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4215402/
- https://www.asco.org/research-guidelines/cancer-progress-timeline/lung-cancer
- https://www.nature.com/articles/s41392-019-0099-9
- https://obamawhitehouse.archives.gov/the-press-office/2016/02/01/fact-sheet-investing-national-cancer-moonshot
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6928196/
- https://www.ncbi.nlm.nih.gov/pubmed/28502785
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6447513/
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









