UK Becomes First Country to Approve Pfizer/BioNTech’s COVID-19 Vaccine
December 2, 2020 – In a major breakthrough in the COVID-19 vaccine race, the Medicines & Healthcare Products Regulatory Agency (MHRA) of the United Kingdom has given an emergency authorization to Pfizer and BioNTech’s COVID-19 vaccine, BNT162b2. With this approval, the UK has become the first country in the world to approve an mRNA vaccine for widespread use. This also marks the first authorization of a COVID-19 vaccine in the western world, following approvals in Russia and China.
“Today’s Emergency Use Authorization in the U.K. marks a historic moment in the fight against COVID-19,” said Albert Bourla, Chairman and Chief Executive Officer of Pfizer. “This authorization is a goal we have been working toward since we first declared that science will win, and we applaud the MHRA for their ability to conduct a careful assessment and take timely action to help protect the people of the U.K.,” he added.
First Approval of an mRNA Vaccine
Vaccine development typically takes years of testing and standardization before reaching the vaccination stage. However, the severity and the unprecedented impact of the COVID-19 pandemic on global health and economy has yielded a vaccine with speed, five times that of a regular approval. BNT162b2 is certainly not the first mRNA-based vaccine to be developed for a disease, but the pandemic has forced it to become the first-ever to receive approval.
The vaccine, which is administered intramuscularly, is a nucleoside-modified messenger RNA encapsulated in lipid nanoparticles. It encodes a mutated form of the SARS-CoV-2 spike protein that elicits an immune response and enables the body to produce protective antibodies against the virus. On November 18th, the final analysis of the Phase 3 trial revealed that BNT162b2 met all of the primary efficacy endpoints and registered a vaccine efficacy of 95% with no safety issues.
“We have carried out a rigorous scientific assessment of all the available evidence of quality, safety, and effectiveness. The public’s safety has always been at the forefront of our minds – safety is our watchword,” said Dr. June Raine, Chief Executive of the MHRA.
Storage and Distribution
However, the biggest challenge for Pfizer would be vaccine storage and distribution. The -70°C and -80°C freezing requirements for BNT162b2 would make distribution an uphill task. In July 2020, Pfizer and BioNTech announced an agreement to supply 30 million doses (later increased to 40 million doses) of the vaccine to the UK, the first of which is expected to roll out in the coming week. Meanwhile, BNT162b2 is also submitted for FDA review in the US, and an emergency approval is on the cards.
“As we anticipate further authorizations and approvals, we are focused on moving with the same level of urgency to safely supply a high-quality vaccine around the world. With thousands of people becoming infected, every day matters in the collective race to end this devastating pandemic,” said Albert Bourla.
Speaking to Sky News, Health Secretary Matt Hancock said: “Fantastic news! The MHRA has clinically authorized the vaccine for rollout. The NHS stands ready to make that happen. So from early next week, we will start the process of vaccinating people against COVID-19 here in this country.”
He further stated that there would be “a combination of three modes of delivery. Fifty hospitals across the country are already set up and waiting to receive the vaccine as soon as it’s approved, so that can now happen. Also, vaccination centers, which will be big centers where people can go to get vaccinated. They are being set up now.”
According to the latest advice of the Joint Committee on Vaccination and Immunisation (JCVI), the following groups would be given priority to vaccination.
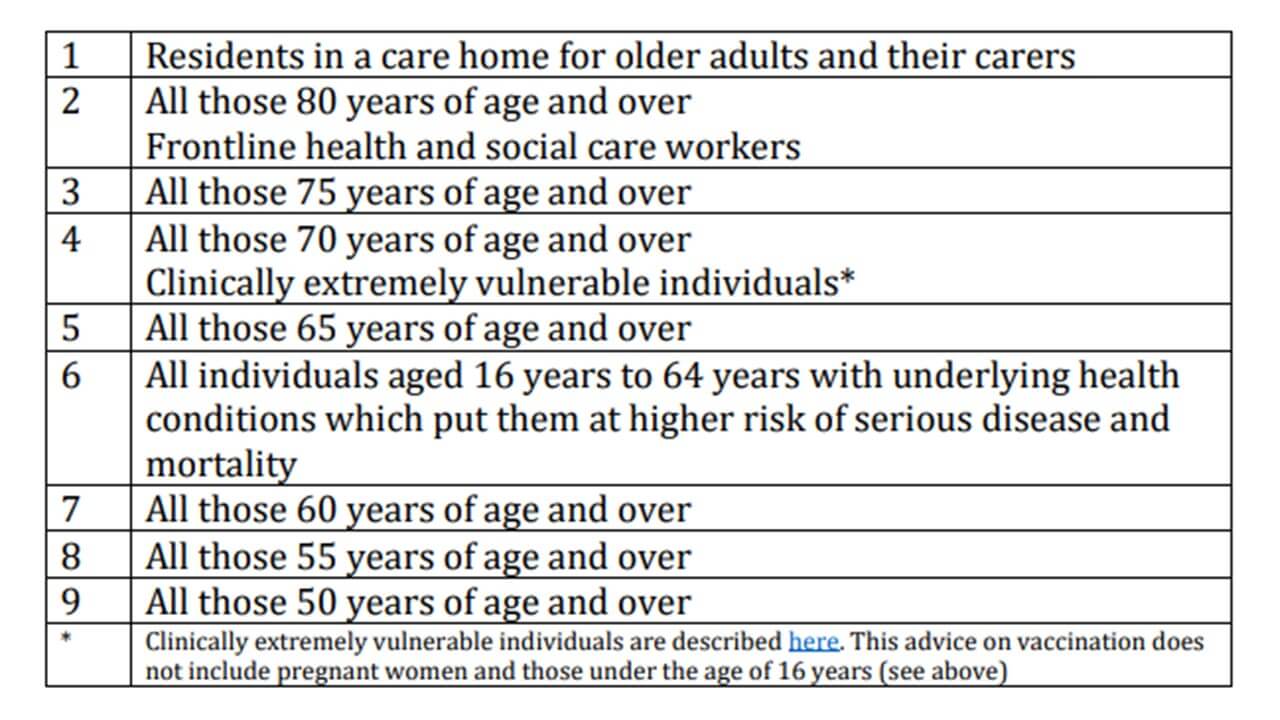 Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination
Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination
By Rajaneesh K. Gopinath, Ph.D.
References
- https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-achieve-first-authorization-world
- https://www.gov.uk/government/news/uk-medicines-regulator-gives-approval-for-first-uk-covid-19-vaccine
- https://news.sky.com/story/covid-19-uk-approves-use-of-pfizers-coronavirus-vaccine-12148786
- https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/940396/Priority_groups_for_coronavirus__COVID-19__vaccination_-_advice_from_the_JCVI__2_December_2020.pdf
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









