Virus-Associated Cancers and Advancements in Their Treatments
By Sangeeta Chakraborty, Ph.D.
Overview
Viral oncology has come a long way since Peyton Rous’s landmark work on chicken sarcomas in the early 1900s at the Rockefeller Institute. His startling discovery that tumors could be transmitted from a sick bird to a healthy one in the same brood, with the filtered cell-free solution of the tumor, proved that cancers could be transmitted by a noncellular agent, probably a virus. Despite the crucial discovery, Rous held off publishing it until much later in 1911 while, just a few years sooner in 1908, two Danish scientists, Ellerman and Bang, published something similar, that filterable agents could transmit leukemia among chickens. Since cancers are not contagious and, avian tumors are widely dissimilar to human cancers, the concept of virus-associated human cancers never gained any traction, and Rous’s contemporaries largely ignored the controversy for almost half a century. But the discovery of Epstein-Barr virus (EBV) in 1953 by Anthony Epstein and Yvonne Barr in the cultured tumor cells from Burkitt’s lymphoma patients changed the way cancers were perceived. Scientists developed a renewed interest in viruses as a possible cause of cancers.
Since Rous’ initial discovery more than a century ago, seven viruses have now been identified to cause invasive cancers in humans. Together they account for 10-15% of all cancer cases worldwide. Despite being responsible for the majority of infection-related cancers, viruses are not the only ones to blame; oncogenic bacterium- Helicobacter pylori and three parasites have also been identified as causative agents of cancers (See Table).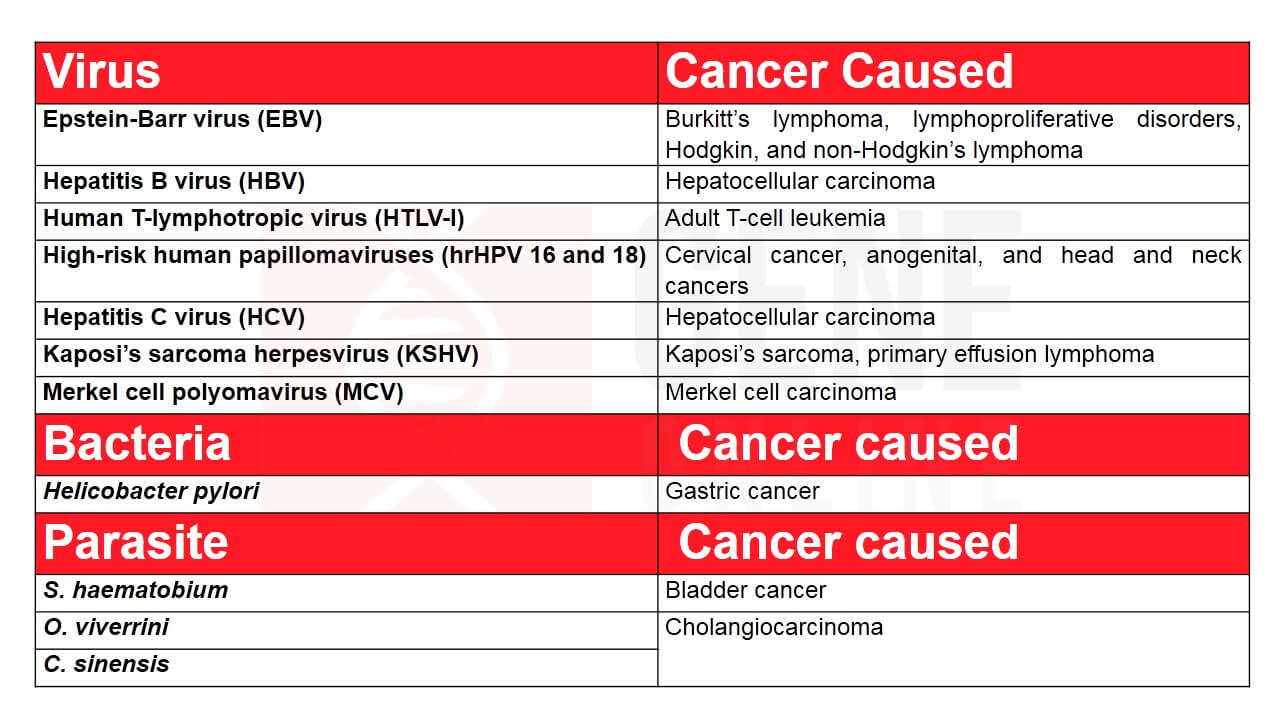
The seven tumor viruses are quite varied in their genome, containing either DNA or RNA, and each has a unique oncogenic mechanism that can alter a normal host cell into a malignant one- a cell that grows progressively. However, it often takes years of persistent infection and inflammation in the host system to set the stage for a cancerous transformation. During this multistep development of a tumor, the virus or the viral components evade immune detection, disrupt cell-cycle checkpoints, modulate the immune system from an attacking state to a tolerant state, and lead to cell immortalization.
Although overly simplistic, the oncogenic mechanisms are either directly or indirectly carcinogenic. As direct carcinogens, EBV, HTLV-I, HPV, KSHV, and MCV either integrate a portion of their DNA into the host cell genome or exist as stable episomes and express viral protein(s) that cause a malignant phenotype. Indirect carcinogens often induce malignant transformation after prolonged and chronic inflammation that deregulates the immune system and potentiates many oncogenic mutations in host cells, for example, in HBV and HCV-related hepatocellular carcinomas, H. pylori, and parasite-mediated cancers. Although considered an indirect carcinogen, in almost all HBV-related hepatocarcinoma, the HBV DNA is usually found integrated into the genome of host cells, but whether specific viral products or the infection and inflammation solely drive the cancer is not clear yet.
Virus-driven oncogenesis may take years to develop after the initial infection or after long periods of viral latency, but the process is often an uncommon and rare secondary consequence rather than a direct outcome of infection.
Prevention and Treatment of Virus-Associated Cancers
From non-specific immunotherapies with interferons and interleukins to targeted therapies with monoclonal antibodies, virus-specific T-cells, and oncolytic viruses, researchers have made substantial headway in the last decade toward prevention and treatment of virus-associated cancers. Since they are caused by infections, these cancers are suitably poised for various prophylactic strategies for prevention that can positively impact their global health burden. Indeed, prophylaxis could bring down the incidence of virus-driven cancers by 19% in developing countries and 3.8% in developed countries.
Prophylactic Vaccines
Prevention or treatment of the specific viral infection should ideally decrease the incidence of the vast majority of virus-associated cancers worldwide. Today, vaccines are available for HBV and HPV. The prophylactic vaccine against HBV consists of Hepatitis B virus surface antigen (HBsAg) in a recombinant vector (mainly the yeast S. cerevisiae). In the last three decades, vaccination against HBV has dramatically brought down infection rates with a corresponding decrease in the incidence of hepatocellular carcinoma. But despite its success, chronic HBV infections still afflicts large populations across the globe.
EBV, a member of the herpes family, currently infects over 90% of the global population. Although currently there is no licensed vaccine available for EBV, the latest research into the development of one has focused on EBV glycoproteins gH, gL, and gp42 that together facilitate viral entry into B-cells and epithelial cells. Jeffrey I. Cohen and Wei Bu, of NIAID, have recently developed nanoparticle vaccines that express either the EBV fusion-machinery protein gH/gL or a combination of gH/gL with gp42. The vaccines were reported to have prevented both epithelial-cell and B cell infection by EBV.
HPV vaccines are subunit vaccines made of the major HPV coat protein L1. Morphologically the protein resembles the virus but has no DNA and, therefore, non-infectious. The thee licensed vaccines currently in use are Cervarix, a bivalent hrHPV16/18 product; Gardasil, a quadrivalent hrHPV16/18/6/11 product; Gardasil 9, a nonavalent hrHPV6, 11, 16, 18, 31, 33, 45, 52, 58 product. HPV vaccinations have not achieved massive success because cervical intraepithelial neoplasia (CIN) (abnormal cellular growth on the surface of the cervix), remains common.
Prophylactic vaccines can only prevent viruses from infecting; they do not prevent the development of cancers. For treating existing cancers, strategies are being developed that can directly attack the virus-transformed malignant cell.
Immune Checkpoint Inhibitors
Persistent viral infections promote the expression of immune checkpoint molecules, such as programmed death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) on immune cells. In a healthy body, these molecules keep T-cell effector functions under check and prevent attacks on self-antigens; however, in virus-associated cancers, the infection triggers a state of sustained expression of these molecules and limit immune-mediated clearance of the virus-laden tumor cells.
Immune checkpoint inhibitors are monoclonal antibodies (Mabs) that block the binding of immune checkpoint molecules to their ligands, relieving the immunosuppressive effect and enhancing the immune effector functions of T-cells. Currently, the immune checkpoint inhibitors approved by Food and Drug Administration (FDA) include anti-PD-1 (nivolumab and pembrolizumab), anti-PD-L1 (atezolizumab, avelumab, and durvalumab), and anti-CTLA-4 (ipilimumab and tremelimumab). A variety of clinical trials are underway testing the efficacy and safety of immune checkpoint inhibitors in virus-related cancers. The anti-PD-1 antibody has been approved in more than ten types of cancers, including melanoma, renal cell cancer, urinary tract cancer, and colorectal cancer. HPV-associated oropharyngeal cancers have shown promising results with checkpoint blockade Mabs.
T-cell Therapy
In adoptive T-cell therapy, viral-specific T-cells are isolated, expanded, and subsequently administered back into a patient, where they can attack the tumor cells that are expressing the specific viral antigen.
In HPV-associated cancers, tumor cells constitutively express two major viral proteins E6 and E7, providing an excellent target for T-cell based therapy that can easily discriminate malignant cells from healthy cells. In 2019, Mei Li Kwong’s group conducted the first Phase I/II study of T-cell receptor therapy for HPV-associated epithelial cancers. Patients received genetically engineered T-cells expressing a T-cell receptor (TCR) directed against HPV16 E6 and eventually showed tumor regression. Several other clinical trials are underway that are utilizing HPV16 E7 TCR expressing T-cells to treat HPV-associated oropharyngeal cancers.
Viral-specific T-cells have also been successfully used in the treatment of EBV-associated cancers. The CD4+ and CD8+ T-cells mainly target the type III EBV proteins like the EBV nuclear antigen (EBNA) 1 and latent membrane protein (LMP) 1, and 2A proteins. In posttransplant lymphoproliferative disease (PTLD) patients, where latent EBV reactivates due to immunosuppression after transplant surgeries, infusion of EBV type III protein-specific T-cells resulted in significant tumor regression.
Oncolytic Virus Therapy
Oncolytic viruses (OVs) are the latest breakthrough in the field of cancer immunotherapy. As the name suggests, these viruses directly kill cancer cells and/or, as research shows, may trigger host innate and adaptive immune responses against cancer too. OVs are engineered to improve their tumor-destroying functions by enabling expression of tumor-specific promotors in their genome, knocking-out virulent viral genes, and introducing cytotoxic and immunomodulatory genes.
In 2015, the FDA approved the first oncolytic virus therapy, talimogene laherparepvec (T-VEC) for advanced melanoma. T-VEC is an oncolytic herpes virus lacking ribonucleotide reductase and expressing granulocyte-macrophage colony-stimulating factor (GM-CSF), combining the oncolytic effects with an immunomodulatory function.
As a monotherapy, oncolytic viruses show limited efficacy, which is why clinical trials currently are combining OVs with either immune checkpoint inhibitors or adoptive T-cell therapies to promote sustained antitumoral responses.
Another issue being, systemic administration of OVs in cancer patients often triggers an antiviral immune response that prevents the virus from reaching tumor tissues. To overcome this, intratumoral injections have been in practice.
No single immunotherapy has had complete success in treating specific virus-associated cancers due to which trials are now focusing on combinatorial immunotherapy.
Editor: Rajaneesh K. Gopinath, Ph.D.
Related Article: Oncolytic Virotherapy – An Overview
References
- https://link.springer.com/article/10.1007/s10739-008-9171-y
- https://link.springer.com/article/10.1007/BF02227892
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4581426/
- https://www.ncbi.nlm.nih.gov/pubmed/20497566
- https://www.cell.com/molecular-therapy-family/oncolytics/fulltext/S2372-7705(19)30097-X
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









