Weekly Cover: LATEST Cell Therapy Trend in Taiwan& 2019-nCoV Outbreak
20200113-0119
LATEST Trends on Cell Therapy in Taiwan
As the “Regulations Governing the Application of Specific Medical Examination Techniques and Medical Devices” and the “Laboratory Developed Tests (LDTs)” were launched in Taiwan at the end of last year, biotechnology companies are increasingly preparing for the unprecedented market.
-TIMELINE-
On December 18, 2019, the Ministry of Health and Welfare passed the first approval of knee cartilage defect cell treatment in Taiwan, based on the newly released “Regulations Governing Specific Cellular Therapeutic Technology”, led by E-DA Hospital and Metatech, On December 21, twenty patients with cartilage defects of the knee joint signed a consent form and were set to begin the process in the near future.
On December 26, 2019, the Ministry of Health and Welfare re-approved three special control law cases, one of which, the collaboration in between EMO biomedicine and Far Eastern Memorial Hospital was approved of the implanting stem cells for degenerative knee arthritis.
On January 14, 2020, Chang Gung Memorial Hospital at Linkou and Unicocell were also approved for products dealing with degenerative knee arthritis and cartilage defects.
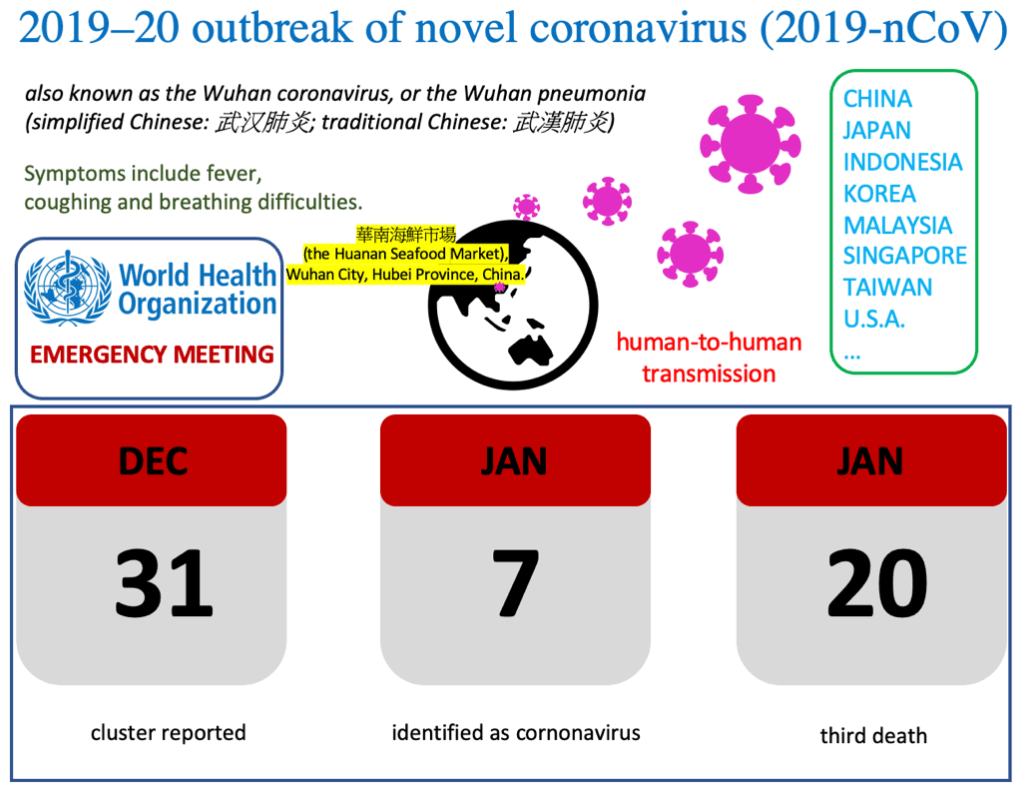
2019–20 outbreak of novel coronavirus (2019-nCoV)
A new SARS-like virus originated in Wuhan, China
is spreading from person-to-person, heightening concern about the risk of wider transmission.
China authorities have reportedly linked a SARS-like coronavirus, Wuhan pneumonia, or 2019-nCoV, to a seafood market located in Wuhan City, Hubei Province, China, suggesting animal-to-person spread at first place.
Zhong Nanshan, a pivotal figure in China’s response to SARS crisis back in 2002, told the press in a news conference on Jan. 21st that the government had not held back information in reporting the outbreak in Wuhan.
Dr. Linfa Wang, virologist at the Duke-National University of Singapore, joined NPR and inferred that the virus is at the moment spreading between humans.
As the number of victims of 2019-nCoV is climbing and spreading worldwide intermittently, at this moment, government officials are on high alert.
The Centers for Disease Control and Prevention (CDC) is closely monitoring this novel coronavirus in its origin, Wuhan City, Hubei Province, China.
The World Health Organization (WHO)
will convene an expert panel on January 22, 2020 to assess
whether the outbreak of the new coronavirus constitutes an international health emergency and what measures should be taken, Reuters reports.
Chinese health authorities posted the full genome of the so-called “2019 novel coronavirus” or “2019-nCoV” in GenBank, the NIH genetic sequence database, as well as the Global Initiative on Sharing All Influenza Data (GISAID).
As of January 22, 2020,
more than ten people died of 2019-nCoV, as the number of infected hit probably more than a thousand globally.
Please visit GeneOnline for updates of the 2019-nCoV outbreak.
Coronaviruses are a large family of viruses, causing illness or circulate among animals, including camels, cats and bats. Animal coronaviruses evolve and infect people and while spreading human-to-human.
About 2019-nCoV
The 2019–2020 novel coronavirus outbreak, also known as the Wuhan coronavirus, or the Wuhan pneumonia, began in mid-December 2019 in Wuhan, China, now transmits human-to human.
Charles River and Takeda Partners to Identify and Develop Preclinical Candidates
Charles River, an American preclinical and clinical laboratory services company, announced on January 13, 2020 a multi-year collaboration with Takeda Pharmaceutical Company Limited, to launch multiple integrated programs focused on drug discovery. Under the alliance, Charles River will leverage its end-to-end drug discovery and safety assessment platform to explore and progress programs toward potential therapeutic candidates. Takeda will consequently advance the preclinical candidates through its clinical development pipeline.
To establish the collaboration, Takeda will pay Charles River a one-time, upfront fee. Charles River will reciprocally be eligible to receive development payments in preclinical and clinical milestones for candidates that progresses to registration.
The agreement also includes additional potential commercial milestones of up to USD 120 million plus royalties on launched products.
About Charles River
Charles River Laboratories, Inc. provides clinical tests and drug development services. The Company offers test and development services for vaccines, biosimilars, and medicine to treat cardiovascular, skeletal, endocrine, central nervous system, cancer, and other sorts of ailments. Charles River Laboratories provides their services globally.
About Takeda
Takeda Pharmaceutical Company is a global, values-based, R&D-driven biopharmaceutical leader headquartered in Japan focusing in R&D on four therapeutic areas: oncology, gastroenterology, rare diseases and neuroscience.

Cyclica and AUM Biosciences to Develop Novel Cancer Therapies
On January 15, 2020, AUM Biosciences (AUM), a Singapore-headquartered clinical-stage biotechnology company committed in the development of affordable cancer therapies, and Cyclica, a Toronto-based biotechnology company, signed a contract to apply Cyclica’s proprietary drug discovery platform in AUM’s diverse R&D programs for the early-stage discovery of novel cancer therapies to decentralize drug discovery.
Collaborated under Project Nexus, leveraging classical drug discovery approaches in search of effective and affordable cancer treatments is the main goal of this strategic partnership.
Under the terms of agreement, AUM will deploy its biomarker-driven drug development approach, and utilize Cyclica’s integrated AI-augmented and structure-based platform, Ligand Design and Ligand Express, to design advanced lead-like molecules with minimize unwanted off-target effects while attaining a holistic understanding of a molecule’s activity.
Cyclica will receive an upfront payment as well as milestone payments upon the completion of specific stages. AUM will maintain rights for future development and commercialization of drug assets.
About Project NEXUS
Project NEXUS is a National Science Foundation (NSF) grant funded project based at the University of Maryland, College Park, launched to investigate innovative models for education.
About AUM Biosciences
Led by a management team with over 100 years experience in the field of oncology drug development, AUM Biosciences (AUM), an oncology-focused Asian biotechnology company, is developing affordable cancer therapies.
AUM is the recipient of Frost & Sullivan’s 2019 Asia-Pacific Biotech Entrepreneurial Company of the Year.
About Cyclica
Cyclica, Inc. is a Toronto, Canada-based biotechnology company decentralizing the discovery of new medicines with its integrated structure-based and AI-augmented drug discovery platform. The Company is striving to minimize off-target effects, and to provide holistic understandings of a molecule dynamics through integrated systems biology and structural pharmacogenomics.
About Metatech
MetaTech (AP) Inc., a Tawian-based semiconductor company, distributes ICs (integrated circuits) components and modules used in communication, computer, and consumer electronics products.
Global Forum “the Cell & Gene Therapy and the Booming China Market“- 38th Annual J.P. Morgan Healthcare Conference
A Short Review and Several Highlights
on the Advances in Cell and Gene Therapy and Opportunities in China
Cancer is the second leading cause of death on a global scale, accounting for an estimated 9.6 million deaths, in 2018, according to The World Health Organization (WHO). Owing to which, the cancer therapy market has witnessed a significant surge over the years. WHO also estimated new cancer cases to raise by 70% in the coming two decades. Furthermore, by 2030, an estimated 4.3 million Chinese may be diagnosed of cancer, as reported by IMS Health.
“The Global Forum on Cell & Gene Therapy and the Booming China Market,” was held on January 14, 2020, at San Francisco, U.S.A, amid the annual JP Morgan Healthcare Conference, one of the largest and most informative healthcare investment symposiums in the industry. The CEO of GenScript Biotech, Frank Zhang, PhD, mentioned that FDA has approved four gene and cell therapies recently, which has brought new hope to patients. Dr. Zhang also pinpointed the rapid growth of drug development business in China.
The Forum also tackled challenges such as mitigating treatment side effects, improving treatment efficacy in solid tumors and scaling up biotechnology manufacturing.
References:
- https://www.genscript.com/biotech-global-forum-2020.html?fbclid=IwAR24lqxAeKG2ftsRL1d02aFCn0i7SIU6Vv3GVoBfBv2q5GZN4EDgBc0cfbQ
- http://www.genetinfo.com/investment/featured/item/33976.html
- https://www.who.int/westernpacific/emergencies/novel-coronavirus
- https://www.nytimes.com/2020/01/15/world/asia/coronavirus-japan-china.html
- https://www.bbc.com/zhongwen/trad/chinese-news-51171551
- https://global.udn.com/global_vision/story/8662/4297858
- https://www.bloomberg.com/news/articles/2020-01-19/wuhan-pneumonia-outbreak-widens-with-china-increasing-screening
- https://www.bloomberg.com/news/articles/2020-01-21/china-virus-s-spread-puts-global-health-officials-on-high-alert
- https://apnews.com/Business%20Wire/3e89378ebce14f9c9c49a7e5bb57f62c?fbclid=IwAR24lqxAeKG2ftsRL1d02aFCn0i7SIU6Vv3GVoBfBv2q5GZN4EDgBc0cfbQ
- https://cyclicarx.com/news/cyclica-and-aum-biosciences-to-partner-on-developing-novel-cancer-therapies-with-greater-precision-and-speed-under-project-nexus
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com









