Weekly in Asia(0428-0503)
Innovent Doses First Patient in IBI318 Bispecific Antibody Clinical Trial in China
Innovent Biologics, Inc., a top-tier biopharmaceutical firm, recently declared that the first patient has successfully been dosed in China in the Phase 1 trial of a recombinant completely human bispecific antibody targeting programmed cell death receptor-1 & programmed cell death ligand-1, a revolutionary antibody developed in collaboration with Eli Lilly & Company. CIBI318A101 is a Phase I clinical study conducted in China to evaluate IBI318 in the treatment of patients with advanced malignancies. The primary objectives of the study are to evaluate the safety, tolerability, and initial efficacy of IBI318.
IBI318 is a recombinant fully human IgG PD-1/PD-L1 bispecific antibody that connects PD-L1-expressing tumor cells and PD-1-expressing T cells, enhancing immune synapses formation, hence potentially augmenting anti-tumor actions and amplifying anti-tumor efficacy. IBI318 is the world’s first PD-1/PD-L1 bispecific antibody to enter clinical development. The development of IBI318 has unique clinical value. This drug candidate may become a more effective treatment option and ultimately benefit more patients.
About Innovent
Established in 2011, Innovent is a Chinese biopharmaceutical company committed to developing, manufacturing and commercializing high quality innovative medicines for the treatment of major diseases in the fields of oncology, ophthalmology, autoimmune, and cardiovascular diseases.
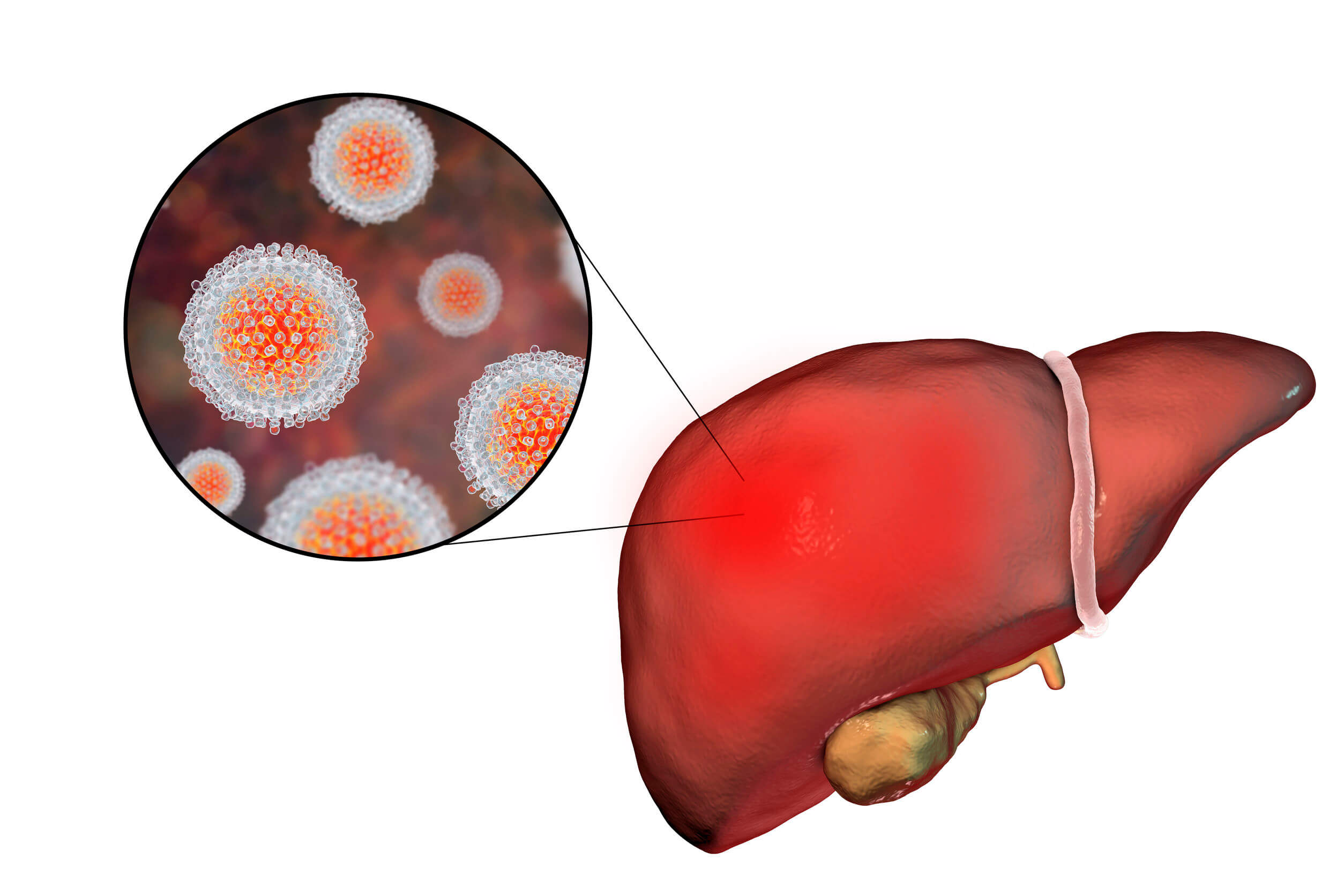
HEC TaiGen Initiates Phase III Trial of a Combination Therapy for the Treatment of Chronic Hepatitis C Patients in China
Hepatitis C is a viral infection that causes liver inflammation.If left untreated, it can sometimes cause serious and life-threatening damage to the liver over years. Current treatments of hepatitis C virus (HCV) in China are imported from abroad and are expensive, which has placed a huge economic burden to patients as well as the healthcare system.
TaiGen Biotech, a Taiwanese infectious disease company, announced on April 23 that they started a China Phase III trial of the combination therapy Furaprevir – Yimitasvir for the treatment of chronic hepatitis C. The combination therapy was developed by Dongguan HEC TaiGen Biopharmaceuticals Co., Ltd, a joint venture established in 2016 by TaiGen Beijing and YiChang HEC ChangJiang Pharmaceuticals. Co., Ltd (HEC). The trial protocol has been approved by Center for Drug Evaluation of NMPA. Three hundred sixty genotype 1 HCV patients will be enrolled. The Completion of Phase III trial is expected in 2020.
About TaiGen Biotechnology
TaiGen Biopharmaceuticals is a leading research-based biotechnology company in Taiwan engaging in the development of novel therapeutics for infectious diseases, cancer research and diabetes-related complications.
CFDA approves lab automation solution by Beckman Coulter 02 May 2019
Clinical laboratories play a crucial role in patient care by delivering to healthcare professionals the diagnostic test results for critical treatment decisions. The faster turnaround time brought by the improvement of laboratory operations and efficiencies enhances the quality of patient care. Consequently, manufacturers of in vitro diagnostics (IVDs) are bringing to the market total solutions to help meet the needs.
The total laboratory automation solution DxA 5000 developed by the global clinical diagnostics leader Beckman Coulter, has recently achieved European CE Mark and China Food and Drug Administration (CFDA) approval. DxA 5000 utilizes a centrifugation protocol that significantly reduces processing time by up to 73%. The system software Intelligent Routing brings automated workflow to the laboratory and continuously calculates the most expeditious route for every patient sample. The automation of sample processing steps helps deliver a higher number of results per hour with the same staffing level.
About Beckman Coulter
Beckman Coulter, Inc. develops, manufactures, and markets diagnostic systems for complex biomedical testing. Its research and discovery products and services covers the fields of diagnostics and life sciences.
Takeda Strikes Value-based Pricing for Crohn’s Disease Gene Therapy in Europe
The growing number and prevalence of treatments such as biologics and gene therapy are worsening the financial burden of healthcare spending. Pharmaceutical companies are now pricing drugs based on performance to keep customers satisfied and also help governments reduce medical expenditures, since cases of ineffective treatments are minimized.
Nikkei Asian Review reported On April 29 that Takeda Pharmaceutical Company is using value-based pricing in Europe for Alofisel (darvadstrocel), an expensive cell therapy used to treat Crohn’s disease. Alofisel is a four-vial treatment course expected to be sold at USD$67,000, which the European Medicines Agency approved last March for patients who do not respond to currently available Crohn’s therapies and may have to receive surgery.
The company is now assessing Alofisel’s profitability under the proposed pricing scheme and will also consider adopting a similar scheme for other treatments in Europe and the U.S.
About Takeda Pharmaceutical Company
Takeda Pharmaceutical Company Limited, together with its subsidiaries, engages in the research, development, manufacturing, and marketing of pharmaceutical products, over-the-counter medicines and quasi-drug consumer products, and other healthcare products. The company provides medicines in various therapeutic areas comprising gastroenterology, oncology, and neuroscience; and vaccines.
Sinovant Sciences Receives Approval of Derazantinib’s Clinical Trial Application by the China National Medical Products Administration
Intrahepatic cholangiocarcinoma (iCCA) is an aggressive cancer that develops in the cells within the bile ducts; both inside and outside the liver. ICCA is one of the greatest unmet needs in oncology, particularly in China, where incidence of cholangiocarcinoma is more than 7 cases per 100,000 people, and a majority of cases are intrahepatic.
Derazantinib is an oral pan-FGFR (fibroblast growth factor receptor) inhibitor being developed as a potential treatment for iCCA and other tumor types with high rates of FGFR mutation.
Sinovant Sciences, a Chinese biopharmaceutical company, has announced that its Clinical Trial Application (CTA) for derazantinib has been accepted by the Center for Drug Evaluation at the China National Medical Products Administration (NMPA). A clinical trial in patients with second-line intrahepatic cholangiocarcinoma (iCCA) will initiate in the second half of 2019.
About Sinovent
Sinovant is a Chinese biopharmaceutical company dedicated to conducting globally innovative biomedical R&D in China to meet the needs of patients in Greater China and around the world.
Reference
1.http://innoventbio.com/en/#/news/140
2.http://www.taigenbiotech.com.tw/NewsDetail/e235f4f5ef8e40e2b6f528675f96e173
3.https://www.biospectrumasia.com/news/28/13361/cfda-approves-lab-automation-solution-by-beckman-coulter.html
4.https://asia.nikkei.com/Business/Companies/Drugmaker-Takeda-to-introduce-value-based-pricing-in-Europe
5.http://www.sinovant.com/d96
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com