Novartis Announces Mayzent Data that Shows Extended Benefit in Secondary Progressive MS
By Rajaneesh K. Gopinath, Ph.D.
Long-term, Phase 3 trial data shows that Novartis’s Mayzent, an FDA approved drug for treating Secondary Progressive Multiple Sclerosis (SPMS), benefits patients by delaying their disability progression for up to 5 years.
Multiple Sclerosis, a Progressive Disease
Multiple Sclerosis (MS) is an inflammatory demyelinating disease that affects the central nervous system (CNS). It is characterized by the body’s immune response against myelin sheath, a fatty substance that wraps spirally around the nerve fibers. This insulating layer facilitates quick and efficient transmission of electrical impulses. However, inflammation, when extended to the nerves, impairs effective neuronal communications.
This chronic disease affects 2.3 million people globally, with 1.2 million in the US and Europe alone. The effects can be different in each patient, with some experiencing mild symptoms like fatigue, memory loss, or blurry vision. Relapse is often the most significant challenge, and therefore, progressive MS could lead to motor skill dysfunction, paralysis, epilepsy, or loss of vision. Relapsing-remitting (RRMS) is the most common type, but others include primary progressive (PPMS) and secondary progressive (SPMS). Around 85% of all diagnosed cases are categorized as SPMS. Additionally, people who exhibit MS-like symptoms but aren’t diagnosed for the disease are classified into a group called clinically isolated syndrome (CIS).
FDA Approved Drugs
The MS drug market is crowded with big players, namely, Novartis, Biogen, Roche, Bayer, Pfizer, Merck, Sanofi, Teva, and GSK, with many of them bagging FDA approvals (See Table 1 & 2). Several drugs are currently under investigation, including Novartis’s ofatumumab, Merck KGaA’s evobrutinib, and Sanofi’s SAR442168. Novartis’ Mayzent is a sphingosine-1-phosphate (S1P) receptor modulator that lowers lymphocyte migration in the CNS. Based on the positive results obtained from Phase 3, EXPAND study, it was approved in March 2019 by the FDA in patients with RRMS and active SPMS (previous progressive-relapsing) subtype.
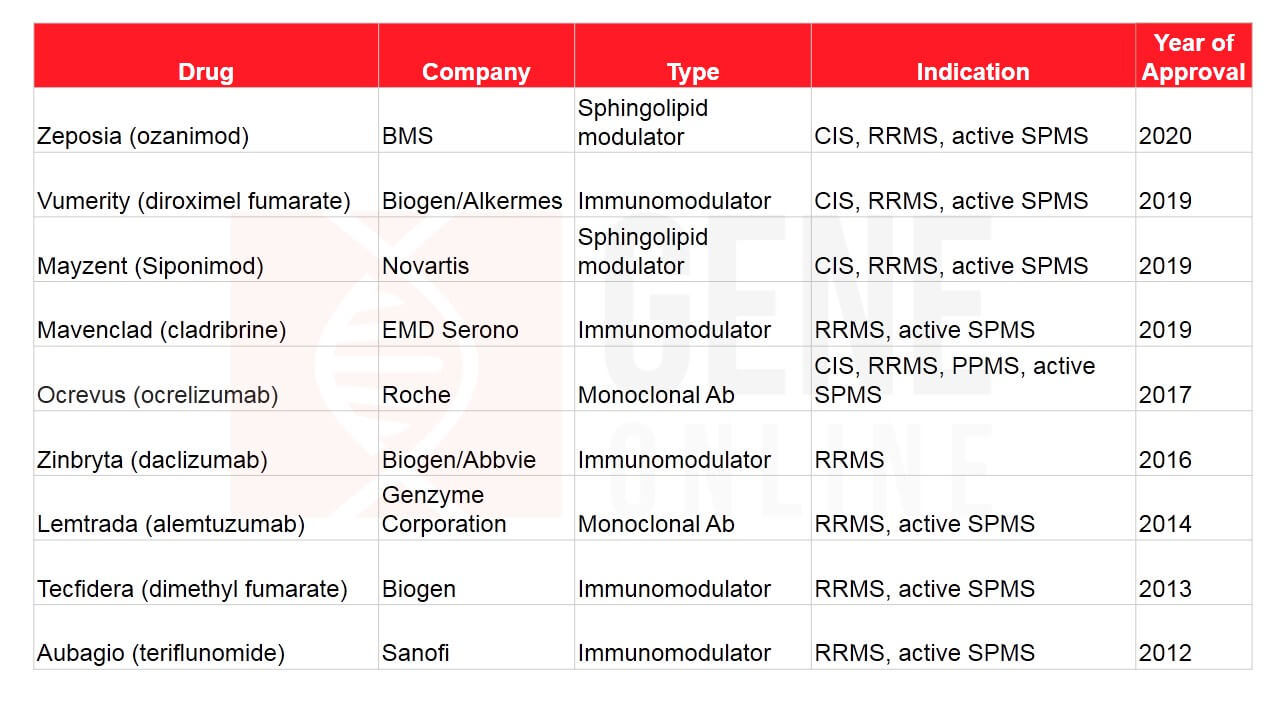 Table 1: FDA approved MS drugs in the past decade
Table 1: FDA approved MS drugs in the past decade
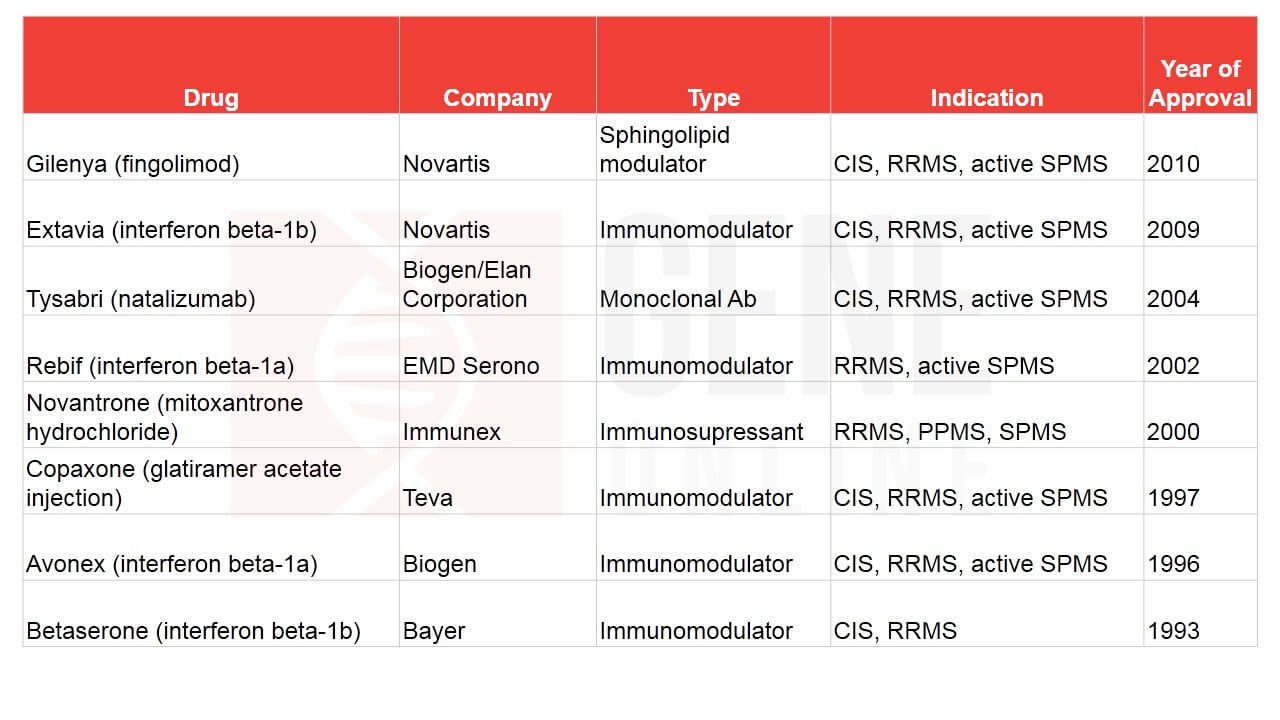 Table 2: FDA approved MS drugs on or before 2010
Table 2: FDA approved MS drugs on or before 2010
Long Term Data from EXPAND Study
The randomized, double-blinded, placebo-controlled, phase 3 trial evaluated Mayzent in 1651 patients from 31 countries who were diagnosed with SPMS. In comparison to the placebo, the Mayzent arm significantly reduced confirmed disability progression (CDP), the primary endpoint of the trial, by 21% (p=0.013). It also achieved secondary endpoints by reducing the annualized relapse rate (ARR) by 55%.
On April 21st, Novartis announced new long-term data of 5 years from the study. Results show that patients who were continuously treated with Mayzent exhibited a lower risk of disability progression and cognitive decline than those who were switched from placebo group to drug treatment. These patients were significantly less likely to experience both three- and six-month CDP, and the ARR was also reduced by 52%. Moreover, Mayzent consistently reduced grey matter atrophy, a hallmark of the disease even in patients who were at a more advanced stage. The results were published in the same issue of the journal Neurology. With this, Mayzent becomes the “first, and only oral disease-modifying therapy (DMT) studied and proven to delay disability progression in a broad range of SPMS patients.”
“These data highlight the critical importance of early treatment intervention with a disease-modifying treatment, such as Mayzent, to ensure the best possible long-term outcomes for patients with MS who are experiencing progression,” said Bruce Cree, MD, Ph.D., MAS, Clinical Research Director, and George A. Zimmermann Endowed Professor in Multiple Sclerosis, UCSF, School of Medicine. “It’s never too early to stay ahead of progression in multiple sclerosis since the early identification of physical and cognitive changes – even subtle ones – can indicate MS disease progression and therefore allow for timely intervention.”
Related Article: Sanofi’s BTK Inhibitor Meets Primary Endpoint in Phase 2 Trial Amidst Criticism
References
- https://www.novartis.com/news/media-releases/novartis-announces-new-mayzent-siponimod-data-show-sustained-effect-delaying-disability-five-years-patients-secondary-progressive-multiple-sclerosis-spms
- https://n.neurology.org/content/94/15_Supplement/4128
- https://n.neurology.org/content/94/15_Supplement/1130
- https://www.drugs.com/history/mayzent.html
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com











