Antimicrobial Peptide Found in Fruit Flies Could Be The Key For New Antibiotic
Scientists at the University of Illinois Chicago have conducted research on fruit flies and discovered that they have a natural defense mechanism against harmful microorganisms. They produce an antimicrobial peptide called drosocin (Dro) that plays a key role in this protection. Dro attaches itself to bacterial ribosomes, hindering their proper functioning and preventing protein synthesis within the cells. This disruption of protein synthesis serves as a defense mechanism against bacterial infections.
Related article: The World’s First mRNA Vaccine to Fight Against Antibiotic-Resistant Bacteria
Dro’s Mechanism Provokes Bacterial Self-Destruction
Dro shares similarities with other peptides that also bind to ribosomes. Although the specific mechanism of Dro’s interaction is not yet fully understood, recent research indicates that it can halt ribosomes at the stop codon, which marks the end of protein synthesis. By doing so, Dro likely prevents the release factor, which is involved in terminating protein synthesis, from properly associating with the ribosome. Essentially, this antimicrobial peptide causes the bacteria to self-destruct. This process is similar to another antimicrobial peptide called apidaecin (Api) found in honeybees, categorizing Dro as a type II PrAMP (peptide with antimicrobial properties).
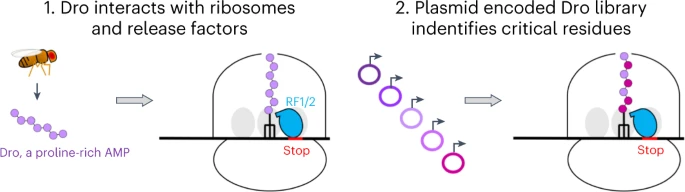
Decoding the Secret Behind Dro Would Propel Antibiotic Development
Further investigation into various mutants of Dro has revealed that its interaction with ribosomes differs from that of Api. In Api’s case, only a few specific amino acids at the end of the peptide are crucial for binding to the target. On the other hand, Dro relies on multiple amino acid residues distributed throughout the peptide. Interestingly, by substituting a single amino acid, the effectiveness of this peptide in targeting ribosomes can be significantly enhanced.
“By understanding how these peptides work, we hope to leverage the same mechanism for potential new antibiotics. Comparing side-by-side the components of the two peptides facilitates engineering new antibiotics that take the best from each,” Mankin said.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com








