FDA Boxed Warning for CAR-T therapy, Affecting Bristol-Myers Squibb, Novartis, Janssen, Gilead Sciences
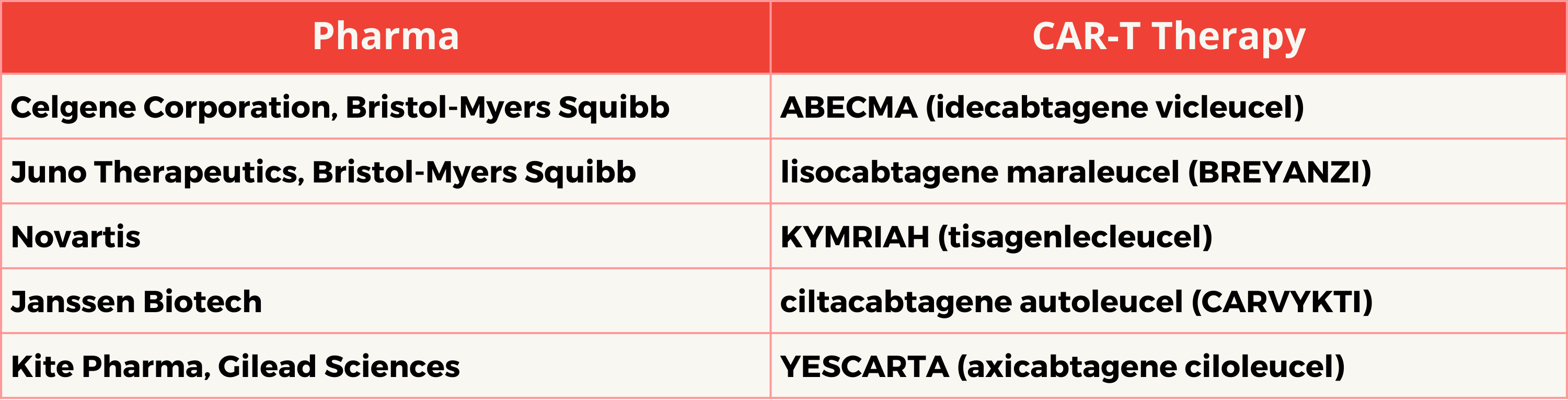
The US Food and Drug Administration (FDA) required a boxed safety warning for five commercial CAR-T cell therapies from Bristol-Myers Squibb, Novartis, Janssen, Gilead Sciences, on January 19, 2024, due to the risk of secondary T-cell malignancies. Boxed safety warning is the most serious safety alert issued by the agency and highlights the potential risk of “secondary T-cell malignancies” associated with these treatments. Despite these risks, the FDA has not banned the use of CAR-T therapy. Instead, it has reminded manufacturers to include a warning about the risks in their product labeling. This warning is intended to ensure that healthcare professionals and patients are fully aware of the risks before making treatment decisions.
What is the risk of secondary T-cell malignancies and why is this a concern?
CAR-T (Chimeric Antigen Receptor T-cell) therapy is a type of immunotherapy that involves genetically modifying a patient’s own T cells to recognize and attack cancer cells. It has shown significant promise in treating certain types of blood cancers, such as leukemia and lymphoma.
The boxed warning emphasizes the risk of developing new cancerous growths of T cells after receiving CAR-T therapy, known as “secondary T-cell malignancies”, which are a group of blood disorders including lymphoma and leukemia. These malignancies can be serious or even fatal, including hospitalization and death, following treatment.
Date back to November 28, 2023, FDA has announced the occurrence of T cell malignancies, including CAR-positive lymphoma, in patients treated with BCMA- or CD19-directed autologous CAR-T cell immunotherapies, raising the awareness of healthcare professionals and patients before making treatment decisions.
Boxed warning alerts to potential risks of CAR-T therapy, calls for medical monitoring
The specific wording of the boxed warning may vary slightly depending on the CAR-T product, but it will generally include the following key points:
- T-cell malignancies have occurred following treatment with CAR-T therapy.
- These malignancies can be mature T-cell malignancies, including CAR-positive tumors, and may present as early as weeks following infusion.
- The malignancies can be fatal.
- Patients who receive CAR-T therapy should be monitored for life for the development of new malignancies.
What should you do if you are considering CAR-T therapy?
If you are still considering CAR-T therapy, it is important to discuss the risks and benefits of the treatment with your healthcare professional. You should also be sure to ask about the specific monitoring plan that will be in place to detect any potential development of secondary T-cell malignancies. The decision of whether or not to undergo CAR-T therapy is different between each individual. It is important to weigh the risks and benefits carefully and make an informed decision in consultation with the healthcare professional.
References
- https://www.fda.gov/media/175622/download?attachment
- https://www.fda.gov/media/175623/download?attachment
- https://www.fda.gov/media/175624/download?attachment
- https://www.fda.gov/media/175625/download?attachment
- https://www.fda.gov/media/175627/download?attachment
- https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- https://www.nature.com/articles/s41591-023-02767-w
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-development-chimeric-antigen-receptor-car-t-cell-products
- https://www.lls.org/car-t-cell-immunotherapy









