Obesity Hope or Hype: Leveraging the Efficacy of Mainstream Weight Loss Pills (First Part)
Obesity is a long lasting and prevalent health issue globally, with significant implications for morbidity and mortality. As such, there is an ongoing need for effective pharmacological interventions to curb this epidemic.
Recent years, from Novo Nordisk to Eli Lilly, the Anti-Obesity Medications (AOMs) trend is taking over the biotech and pharmaceutical industry. Furthermore, these two pharma companies are also the top two pharmaceuticals according to the market capitals.
With The Surge of Anti-Obesity Medications, Comes Up With The Efficacy And Safety Issues
Novo’s Ozempic, a diabetes drug commonly prescribed off-label for obesity, generated revenue of $3.2 billion, up from $2.1 billion the prior year. On the other hand, Lilly’s growth is partly attributed to the sales of Mounjaro, another diabetes drug with potential for obesity treatment, which bolstered Lilly with $980 million in sales in the quarter. Besides, several other drugs have been developed to target different mechanisms involved in appetite control and weight regulation.
However, few people know the actual efficacy of those weight loss pills. Do they really make you slim down? Are those drugs safe? This article review aims to wrap up the most concerning issues of AOMs, from their efficacy, advantages, and also the cost, including the mainstream type of weight loss pills.
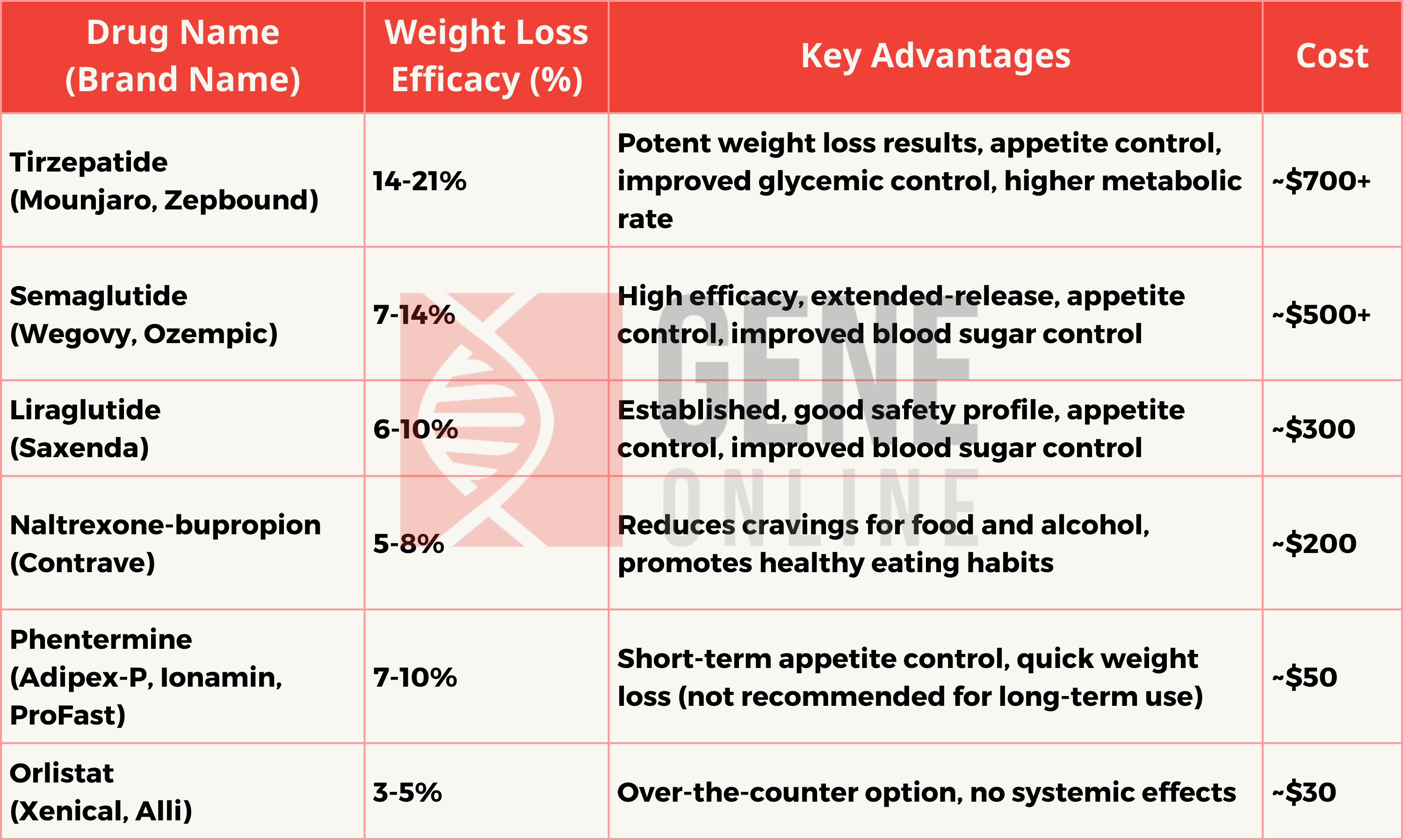
Tirzepatide
In a clinical trial named SURMOUNT-2, Tirzepatide demonstrated a weight loss efficacy of up to 15.7% in adults who are obese or overweight and have type 2 diabetes. This study was a multicenter, randomized, double-blind, parallel, placebo-controlled trial aimed at comparing the effects and safety of Tirzepatide 10 mg and 15 mg versus placebo as an adjunct to a low-calorie diet and increased physical activity in adult patients who are obese or overweight.
The primary objectives of the trial were to prove that Tirzepatide 10 mg and/or 15 mg is superior in terms of the mean percentage change in body weight from baseline and the percentage of participants achieving ≥5% body weight reduction at 72 weeks compared to placebo.
Semaglutide
According to various studies, Semaglutide has demonstrated significant effectiveness in weight loss. In one study, participants using Semaglutide experienced an average weight loss of 15% over 68 weeks, with nearly one-third of the participants losing 20% of their body weight. In another study focusing on non-diabetic patients, Semaglutide was effective in promoting weight loss, with an average weight reduction of 11.85% compared to placebo.
It’s important to note that these studies indicate significant weight loss results with Semaglutide use, but there might be a regain of weight after discontinuation of the drug. Additionally, Semaglutide is typically used in conjunction with diet and exercise for optimal results and is not a standalone weight loss medication.
Liraglutide
In practical applications, the weight loss effect of Liraglutide 3.0 mg is quite significant. One study showed that more than half of the patients using Liraglutide achieved at least a 5% weight loss.
Another research report from Switzerland indicated that using Liraglutide 3.0 mg could lead to significant changes in weight and body composition, with more pronounced effects in female patients. These studies suggest that Liraglutide can not only aid in weight loss but also has benefits for improving body composition.
Naltrexone or Bupropion
The combination medication of Naltrexone and Bupropion has shown quite impressive results in weight loss. In a series of randomized controlled trials, patients treated with a combination of 32 mg Naltrexone and 360 mg Bupropion extended-release (NB32) experienced an average weight loss of 5.0-9.3% over a 56-week treatment period, compared to a weight loss of 3.2-5.2% with placebo. Furthermore, 45-66% of treated patients achieved at least a 5% weight loss, in contrast to 23-34% with placebo.
Additionally, Contrave® (a combination of Naltrexone hydrochloride extended-release and Bupropion hydrochloride extended-release) has been approved for the treatment of obesity, to be used alongside lifestyle modifications. In four randomized, double-blind, placebo-controlled phase III clinical trials, Naltrexone/Bupropion treatment resulted in statistically significant and clinically meaningful weight loss compared to placebo, with an average baseline weight reduction of about 11-22 pounds (approximately 5-9 kg).
Overall, the combination of Naltrexone and Bupropion appears to be an effective adjunctive method for achieving weight loss and treating obesity-related comorbidities. However, the use of such medication may come with some side effects such as nausea, constipation, headache, and vomiting. In most patients, treatment with NB32 was well tolerated. Patients should have a detailed discussion with healthcare professionals before using these medications to ensure safety and effectiveness.
Notice and Disclaimer
Individual responses to this medication can vary, so it’s advised to discuss in detail with a healthcare professional before use. These outcomes are based on specific clinical trials and studies, so actual effects may vary from person to person.
References
- Aronne, L. J. et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 331, 38–48 (2024).
- https://investor.lilly.com/news-releases/news-release-details/tirzepatide-demonstrated-significant-and-superior-weight-loss
- https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=166303
- Clinical Efficacy of Orlistat Therapy in Overweight and Obese Patients With Insulin-Treated Type 2 Diabetes | Diabetes Care | American Diabetes Association. https://diabetesjournals.org/care/article/25/6/1033/21377/Clinical-Efficacy-of-Orlistat-Therapy-in.
- Chanoine, J.-P., Hampl, S., Jensen, C., Boldrin, M. & Hauptman, J. Effect of Orlistat on Weight and Body Composition in Obese AdolescentsA Randomized Controlled Trial. JAMA 293, 2873–2883 (2005).
- Garvey, W. T. et al. Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care 43, 1085–1093 (2020).
- Santini, S. et al. Marked weight loss on liraglutide 3.0 mg: Real-life experience of a Swiss cohort with obesity. Obesity (Silver Spring) 31, 74–82 (2023).
- Apovian, C. M. Naltrexone/bupropion for the treatment of obesity and obesity with Type 2 diabetes. Future Cardiol 12, 129–138 (2016).
- Wilding, J. P. H. et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine 384, 989–1002 (2021).
- Phentermine weight-loss medicine: Know the side effects. Mayo Clinic https://www.mayoclinic.org/healthy-lifestyle/weight-loss/expert-answers/phentermine/faq-20057940.
- Lewis, K. H. et al. Safety and Effectiveness of Longer-Term Phentermine Use: Clinical Outcomes from an Electronic Health Record Cohort. Obesity (Silver Spring) 27, 591–602 (2019).
- Novograd, J., Mullally, J. & Frishman, W. H. Semaglutide for Weight Loss: Was It Worth the Weight? Cardiology in Review 30, 324 (2022).
- Christou, G. A. & Kiortsis, D. N. The efficacy and safety of the naltrexone/bupropion combination for the treatment of obesity: an update. Hormones (Athens) 14, 370–375 (2015).









