Pioneering Trial for Hearing Loss Medication Yields Promising Insights Despite Falling Short in Results
A world-first trial for a hearing loss medication conducted by researchers from University College London (UCL) and University College London Hospitals NHS Foundation Trust (UCLH) has unveiled valuable insights into hearing restoration. The trial, known as REGAIN (REgeneration of inner ear hair cells with GAmma secretase INhibitors), focused on evaluating the efficacy of LY3056480, a gamma-secretase inhibitor (GSI) in restoring hearing among adults with mild to moderate hearing impairment across the UK, Germany, and Greece.
Contrary to initial expectations, the drug did not demonstrate a significant overall restoration of hearing across the patient group. However, in-depth analysis revealed promising signals of activity in the inner ear among certain individuals. Despite the primary outcome not being met, these findings emphasize the potential of LY3056480 for further development in treating inner ear conditions.
Related article: Overcoming Aging Related Hearing Loss- A Possibility In The Not So Distant Future
GSI May Bring a Ray of Hope for Hearing Loss Patients
Sensorineural hearing loss (SNHL) is often the result of age-related progressive damage or loss of inner ear sensory hair cells and/or their synapse. Because in humans these hair cells do not naturally regenerate, the condition generally progresses with age. Besides, damage to the auditory nerve or the brain’s central processing centers may also lead to such permanent hearing loss. Given its debilitating and irreversible nature and large patient population (more than 360 million people worldwide, according to the World Health Organization), SNHL presents significant unmet medical needs around the world.
Nevertheless, according to the official website of the REGAIN project, recent studies in animals with hearing loss have shown that new and functioning hair cells can be generated through local treatment with a certain GSI and improved hearing, meaning that this family of small-molecule drugs may bring a ray of hope for SNHL patients.
Key Research Findings and Implications of the REGAIN Project
Published in Nature Communications on March 1, the research paper elucidates the methodology and outcomes of the REGAIN trial. The proof-of-concept phase 1/2a multiple-ascending dose open-label trial involved three intratympanic injections of GSI LY3056480 administered to adult patients with mild-moderate SNHL in one ear over a period of two weeks.
The primary focus was on evaluating safety and tolerability in Phase 1 and assessing changes in average pure-tone air conduction threshold at various frequencies in Phase 2a. Results from the Phase 1 trial indicated that LY3056480 delivery via intratympanic injection was safe and well-tolerated, with no severe or serious adverse events reported among the 15 participants. However, in Phase 2a which involved 44 participants across three sites, the drug did not demonstrate a significant improvement in the average pure-tone threshold across 2, 4, and 8 kHz at 6 and 12 weeks post-treatment. Secondary measures, including Distortion Product Otoacoustic Emissions (DPOAE) amplitudes, Signal to Noise Ratios (SNRs), Speech Reception Thresholds (SRTs), and Hearing Handicap Inventory for Adults/Elderly (HHIA/E) scores, also did not show significant changes from baseline.
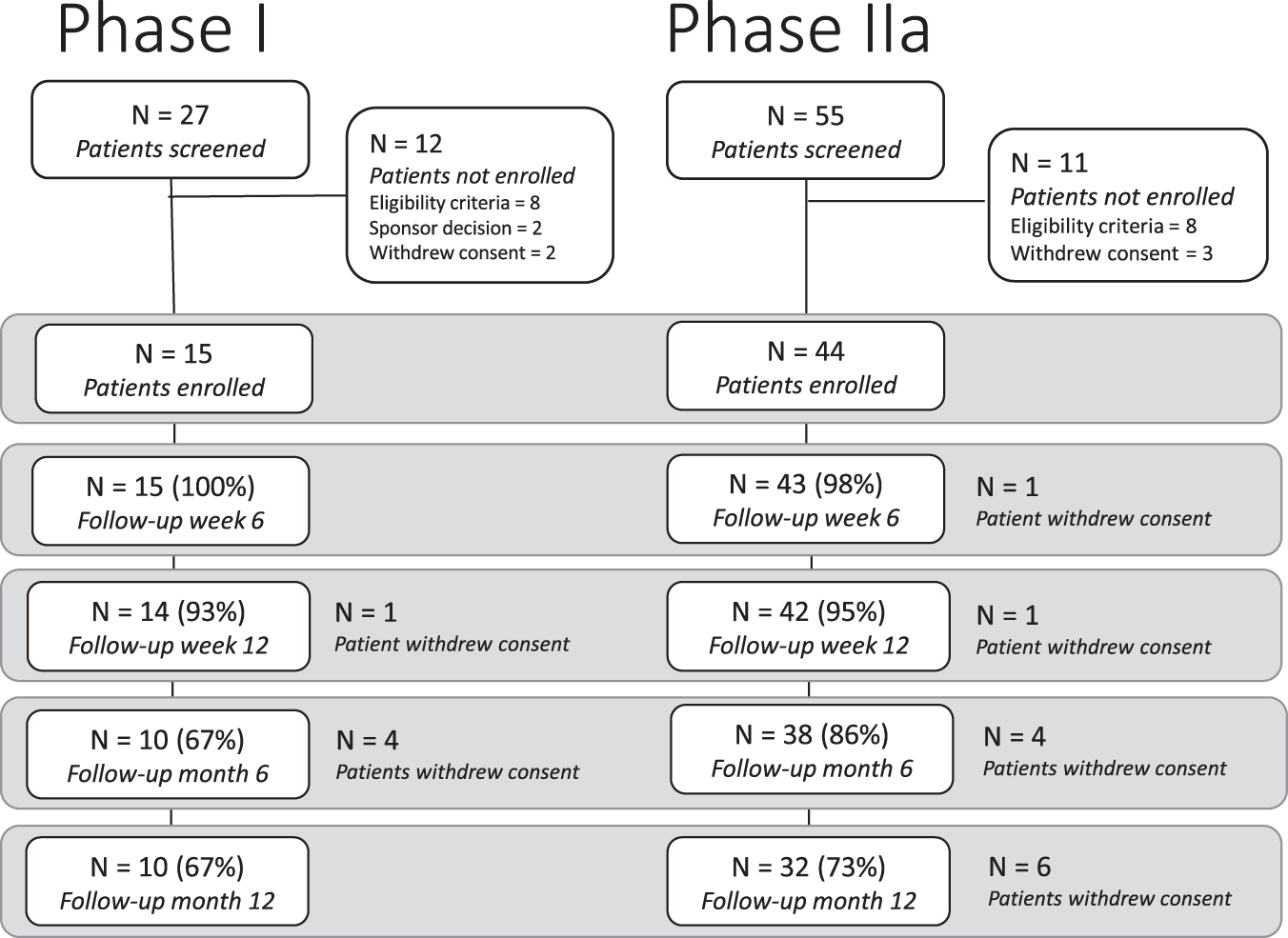
In short, while the trial confirmed the safety and tolerability of intratympanic delivery of GSI LY3056480, it did not meet its primary endpoint of restoring hearing as expected.
Valuable Insights into Future Directions and Collaborative Efforts
Led by Dr. Anne GM Schilder, Professor of Otorhinolaryngology at UCL Ear Institute and Director of the National Institute for Health Research (NIHR) UCLH Biomedical Research Centre Hearing Theme, the REGAIN trial involved an EU Consortium comprising clinical partners from Germany and Greece, along with Audion Therapeutics BV, a Dutch biopharma company. This groundbreaking project, funded by an EU Horizon 2020 grant of €5.8 million ($6.3 million), marks a milestone in hearing loss research for being the world’s first study of a regenerative hearing drug.
Despite the primary endpoint not being met, Prof. Schilder indicated that the study provided valuable insights into future studies of its kind. “For example, the study will help how we best select the patients that may benefit from these new and highly targeted hearing treatments. This requires a better understanding of the mechanisms behind inner ear hearing loss and better hearing tests to identify its causes in patients. Big data and AI may speed up this process,” said Prof. Schilder.
To advance this research, the team has initiated the NIHR Hearing Health Informatics Collaboration (HIC) and established a patient registry named HEDGE, aiming to uncover genetic and environmental factors contributing to hearing loss. These concerted efforts underscore the commitment of researchers and clinicians to advance the understanding and treatment of hearing disorders, paving the way for future breakthroughs in auditory healthcare.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com








