Revolutionary AI-Powered Respiratory Monitor Maker Gets Financial Boost with Latest Funding
Respiratory monitoring has emerged as an indispensable tool for patient care, especially in outpatient settings where procedures such as endoscopy are routinely conducted. In such settings, patients receive sedation outside of the traditional operating rooms. These cases, more commonly known as the Non-Operating Room Anesthesia (NORA) cases, have significantly risen in number, leading to a greater focus on procedural sedation.
While NORA-sedation offers multiple benefits, recent research indicates increased hypoxia (lack of oxygen) or inadequate ventilation events in patients, in addition to a higher frequency of severe injury or mortality. Therefore, highly sensitive and continuous breathing detection systems have become imperative for effective respiratory monitoring.
Traditional stethoscopes are limited in their capabilities as they lack continuous monitoring features. Capnography, which monitors carbon dioxide in exhaled breath, is the current standard of care in general anesthesia. However, emerging evidence shows that it does not measure breathing rates accurately.
Heroic Faith Medical Science, a Taiwan-based medical device company has come up with a solution. Its revolutionary Artificial Intelligence (AI)-powered Respiratory MOnitoring Device or Airmod is a lightweight, non-invasive device that utilizes an FDA-cleared active noise cancellation electronic stethoscope. This innovative technology enables real-time monitoring of alterations in patients’ breathing sounds and converts it into a visual time-sensitive map. The AI adeptly filters out extraneous noise, retaining only clinically relevant breathing sounds and breathing patterns, to deliver invaluable insights for patients and medical professionals.
At the recently concluded 77th PostGraduate Assembly in Anesthesiology (PGA77) in New York, Heroic Faith hosted a thought-provoking symposium on the importance of sedation safety. Soon after the product launch symposium, the company closed an extension round of fundraising with support from internal investors and undisclosed new venture capitalists.
Airmod’s Broad Spectrum of Applications
The Airmod has two components, the Accursound AS-101 electronic stethoscope and the Airmod software. The Airmod and electronic stethoscope has received medical device regulatory clearance from the Taiwan FDA. In 2023, Airmod also obtained 510(k) Clearance from the US FDA.
Airmod is the only method currently available for the early detection of abnormal Partial Airway Obstruction (PAO) sounds under intravenous general anesthesia (IVGA). It can confirm that the patient is ventilating well and is preventing hypoxia caused due to apnea.
Airmod has gained widespread acceptance, being implemented in almost half of the medical centers across Taiwan. Its versatile applications extend from gastroscopy and colonoscopy to pediatrics and plastic surgeries, showcasing its adaptability and effectiveness.
Additionally, Heroic Faith is actively collaborating with ten dental training centers in the US, with the objective of seamlessly incorporating Airmod into specialized fields like pediatric and special needs dentistry.
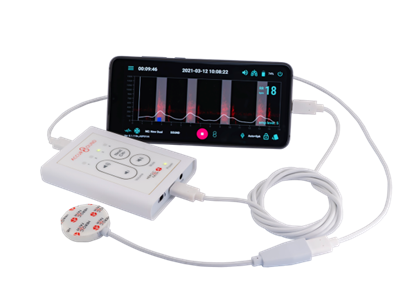
An AI-Powered Solution to Overcome Procedural Sedation Challenges
At the symposium, Dr. Alparslan Turan, Vice Chair of the Outcome Research Center at Cleveland Clinic, highlighted the challenges surrounding procedural sedation and stated that devices that monitor ventilation and oxygenation in patients is a possible solution.
Currently, capnography is widely used as it decreases the number of hypoxic events in patients. However, recent studies show that it does not reduce clinically significant adverse events. Furthermore, its accuracy diminishes when the patient is mouth breathing.
Dr. Turan opined that the Airmod is a good addition to the field and together with capnography, could probably be useful in preventing perioperative events in the future.

Airmod’s Non-inferiority to Traditional Capnography
The symposium also featured Dr. Chien-Chung Huang from Taiwan’s MacKay Memorial Hospital who presented exciting study results that affirm the Airmod’s precision in estimating respiratory rates when compared to traditional capnography.
A total of 151 adult participants were enrolled in a multicenter single-arm observational study conducted in Taiwan. Data collected from 133 participants underscored Airmod’s equivalence in respiratory rate and apnea detection capabilities. Notably, the Airmod system exhibited a significantly lower rate of instrument failure during breath monitoring, distinguishing it from traditional capnography.

In conclusion, Airmod represents a possible solution to the challenges associated with procedural sedation. Airmod addresses critical issues such as hypoxia and inadequate ventilation and would prove to be impactful in the precise detection of respiratory abnormalities.
With the newly secured funding, Heroic Faith is committed to working diligently and capitalizing on the rapid growth trend of medtech AI. Its mission is to ensure that their AI-powered innovation, Airmod, continues to revolutionize procedural sedation and enhance patient safety across diverse medical settings.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com








