Grand Opening of BIO Asia-Taiwan 2023 Showcases the Vitality of Taiwan’s Emerging Biotech Industry
Co-organized by the Biotechnology Innovation Organization (BIO) and the Taiwan Bio Industry Organization (Taiwan BIO), BIO Asia-Taiwan 2023 was grandly held at the Taipei Nangang Exhibition Center from 26 to 30 July.
With the theme of “Embracing Asian Dynamics”, this annual event for the Asian biotech industry boasted a scale of exhibition and conference that surpassed the pre-COVID-19 level, culminating in a record-breaking level of international participation. In addition to exhibitors from 19 countries, a total of 22 sessions of international forums were held during the event, attracting biotech professionals from more than 30 countries.
Related article: Taiwan’s Biotech Brilliance Shines at BIO 2023: Unveiling Cutting-Edge Discoveries and Strategic Partnerships
BIO Asia–Taiwan International Conference Launched to Promote Cross-National Cooperation
The BIO Asia-Taiwan International Conference 2023, held from July 26 to 28, marked the grand opening of the event. Dr. Johnsee Lee, Chairman of BIO Asia–Taiwan 2023, mentioned in his opening speech that the event focused on three major aspects: international investment trends, innovative technologies and therapies, and the marketing supply chain. Besides, about 150 local and international biopharma experts participated in various sessions during the 3-day conference, creating a platform for global biotech industry players to exchange ideas and connect with each other.
Other highlights of this year’s event included the BIO Asia-Taiwan Exhibition with record-high 2,000 exhibitor booths. The event also included seven Regional Collaboration Forums and more than 1,800 1-on-1 business matchmaking sessions in order to foster cross-border business collaborations.

In his speech, Dr. Chung-Hsiun Wu, Chairman of the Taiwan Bio Industry Association (Taiwan BIO), said that the biotech ecosystem in Asia is developing rapidly, and the experience of the COVID-19 pandemic has proved that Taiwan is able to effectively utilize its technology and resources to respond to the emergency needs. He expressed his conviction that BIO Asia-Taiwan could become a platform to promote international exchanges and cooperation, and to gather diversified perspectives in order to jointly shape a bright future for the biotech industry.
Susanna Ling, BIO’s Vice President (Industry Programs and Partnerships), began her speech by introducing the formation and role of BIO and sharing key highlights from the BIO 2023 Convention held in Boston last month. She also highlighted the business matching events organized during this year’s event, in which approximately 650 representatives from 350 biotech companies would be attending and more than 700 meetings had already been scheduled to take place.
Dr. Yi-Juang Chern, Deputy Minister of the National Science and Technology Council, mentioned that the CDMO (Contract Development and Manufacturing Organization) industry would be one of the main focuses of the event. In addition to the international experts sharing the latest global trends, the CDMO zone would again be established during the exhibition this year, with its scale being one of the largest among all the exhibition zones.
Far-reaching Implications of Changes in Biotechnology Policies
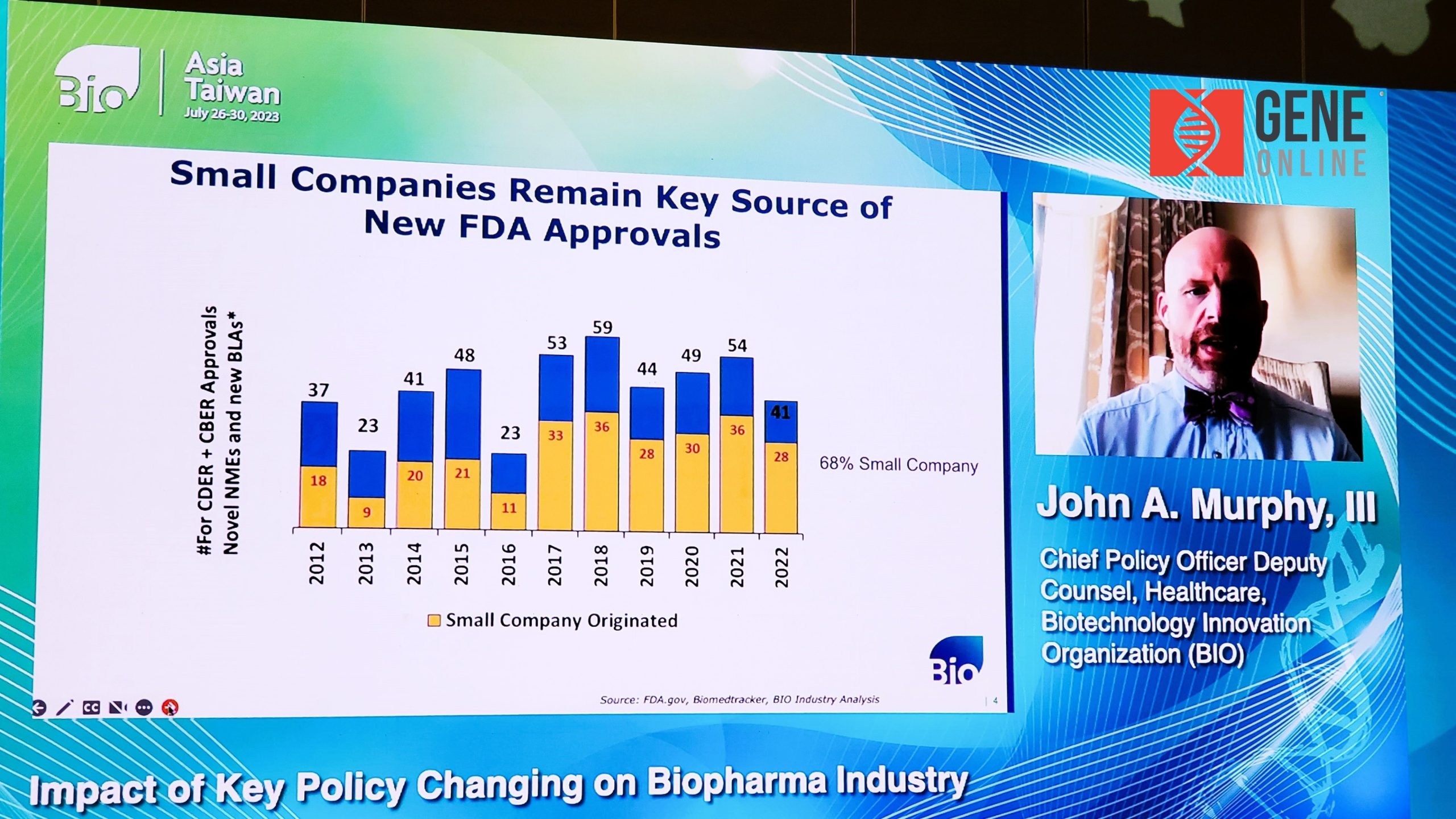
The plenary session began with a keynote speech by John A. Murphy III, BIO’s Chief Policy Officer, on the topic of “Impact of Key Policy Changing on Biopharma Industry”. Although he was unable to attend the conference in person due to the typhoon, he shared his insights on the evolution of government biotech policies in recent years and his expectations for the future in an online format with four key points.
First, small biotech companies have made significant contributions to driving biotech innovations. Last year, for example, about 68% of FDA-approved new drugs came from small companies; second, the U.S. government’s Inflation Reduction Act has been hurting biopharma companies. With the government’s involvement in drug pricing control, it could adversely affect the revenues of pharma companies for the foreseeable future.
Thirdly, President Biden signed an executive order last September to encourage the development of biotechnology and biomanufacturing industries, and the speaker stated that this policy would lead to an acceleration in industry development and a reorganization of the supply chain to reduce foreign dependence. Lastly, he pointed out that while sharing patents spurred by the COVID-19 pandemic would benefit low-income countries by enjoying the fruits of R&D endeavors (e.g. mRNA vaccines), it could also dampen the incentives of biotech companies for investment and prevent technological advancements.
Tang Prize Laureates Invited to Sharing of Research Experiences
Furthermore, during the plenary session, the organizers had the honor of inviting Pieter Cullis and Katalin Kariko, recipients of the 2022 Tang Prize in Biopharmaceutical Science, to deliver presentations online. The former featured applications of lipid nanoparticles in the development of gene therapy, while the latter centered on research and development of mRNA therapies and vaccines.
Sam Wilson, Senior Vice President and Head of APAC (Commercial Solutions) of Syneos Health, was also invited to provide insights into the major trends in the healthcare industry in 2023, highlighting the digital transformation that has revolutionized the way patients and healthcare professionals connect and communicate, thereby improving patient recruitment for clinical trials. He also noted that healthcare leaders are evolving from functional specialists to outcome-driven strategic planners. Another important trend is the shift in focus of M&A strategies, with start-ups and smaller companies turning to outsourcing for cycles of experience and critical decisions related to sustainable growth, which, in the case of Japan, has become an attractive market strategy due to its lower initial investment, operational flexibility and quality.