Efficient Phage Production System Promises Win Against Superbugs
Antibiotic resistance (AMR) occurs when bacteria evolve and stop responding to medicines, making infections more difficult to treat and increasing the risk of spread, severe illnesses, and death. Antibiotics and antimicrobials stop working as a result of resistance, making it harder or impossible to treat illnesses. A promising alternative, phage therapy, has an extensive history of successful application in antimicrobial-resistant or superbug infection cases.
Researchers from Munich published the results of a rapid phage manufacturing platform for treating infection of antibiotic-resistant bacteria, instead of culturing the pathogenic bacteria host to amplify the phages, the team introduced a cell-free phage amplification and engineering method to reduce contamination and speed up the process.
Currently, high contamination rate, need for large volume cultivation of human pathogens, imprecise manufacturing standards, and lack of sufficient quality controls are the factors impeding the widespread adoption of phage therapy for treating superbug infections.
Related article: UK Launches World-First Program With Two Antimicrobial Drugs to Counter Superbugs
Cell-Free Pipeline to Generate Phages Without Bacterial Hosts
Conventionally, to get the proper amount of phages, pathogenic bacteria are first cultured and then exposed to phages. After the phages multiply in the bacteria, they are harvested and purified through complex and expensive processes. The team noted that sometimes, bacterial cell walls contain endotoxins that need to be removed during the purification of phages.
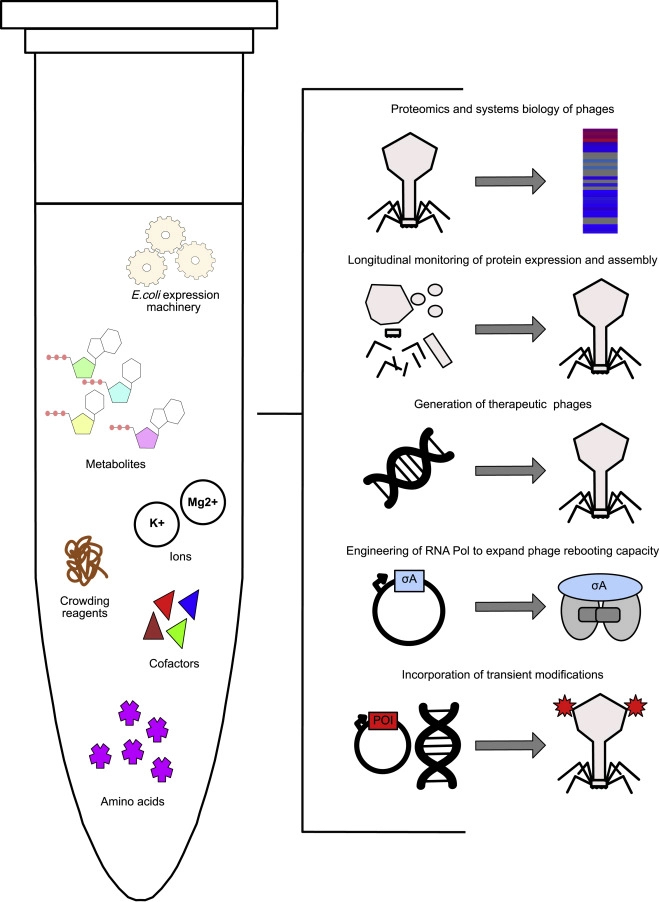
Here, phages are produced in a cell-free environment containing the extracts from destroyed Escherichia coli cells. Coupled with a characterized phage proteome, the researchers can track phage assembly by time-resolved mass spectrometry, effectively monitoring protein expression. The resulting phages are demonstrated to be effective against target bacterial pathogens such as K. pneumoniae.
“At the microliter scale, a one-pot reaction enables the production of therapeutic phages against multidrug-resistant bacteria in a matter of hours at clinically relevant titers,” the team said.
Since the system also allows easy genetic manipulation, researchers engineered phages at the protein level to express polyhistidine-tag (His-tag) capsid protein, allowing easy purification by affinity chromatography. The technique gives researchers and clinicians the ability to modify/tune the phages to boost their power to eliminate target bacteria.
Through advancements in synthetic biology, this cell-free expression platform may be used for generating a diverse array of phages, engineered and produced from synthetic genomes, to tackle different AMR bacteria.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com










