GeneOnline’s Weekly News Highlights: Oct 16-Oct 20
GeneOnline’s editorial team has compiled a digest of top international biotechnology and healthcare news of the week to help readers keep abreast of global biomedical industry updates.
Related article: Daiichi Sankyo Advances Care Standards with Groundbreaking Cancer Treatment
AI-Powered Pain Recognition System Revolutionizes Patient Care
An automated pain recognition system utilizing artificial intelligence (AI) is emerging as a promising approach to identifying and managing pain in patients undergoing surgery, according to research unveiled at ANESTHESIOLOGY 2023, the American Society of Anesthesiologists (ASA) Annual Meeting. Developed by researchers at the University of California San Diego (UCSD), the system combines two forms of AI: computer vision, which provides the computer with the capability to “see,” and deep learning, enabling it to interpret visual cues for pain assessment. This innovative system marks a significant shift from conventional subjective methods of pain assessment to a more accurate and objective approach.
Novo Nordisk Acquires Ocedurenone with $1.3B for Hypertension and Cardiovascular Disease
On October 16, Novo Nordisk A/S and Singapore-based KBP Biosciences PTE., Ltd. announced that the two companies have reached a strategic agreement, with Novo Nordisk acquiring Ocedurenone, a promising oral medication for uncontrolled hypertension, and potential applications in cardiovascular and chronic kidney diseases. This acquisition, to be completed before the end of 2023, represents a substantial step of the Danish pharma giant in addressing a pressing global health concern, with a financial commitment of up to $1.3 billion.
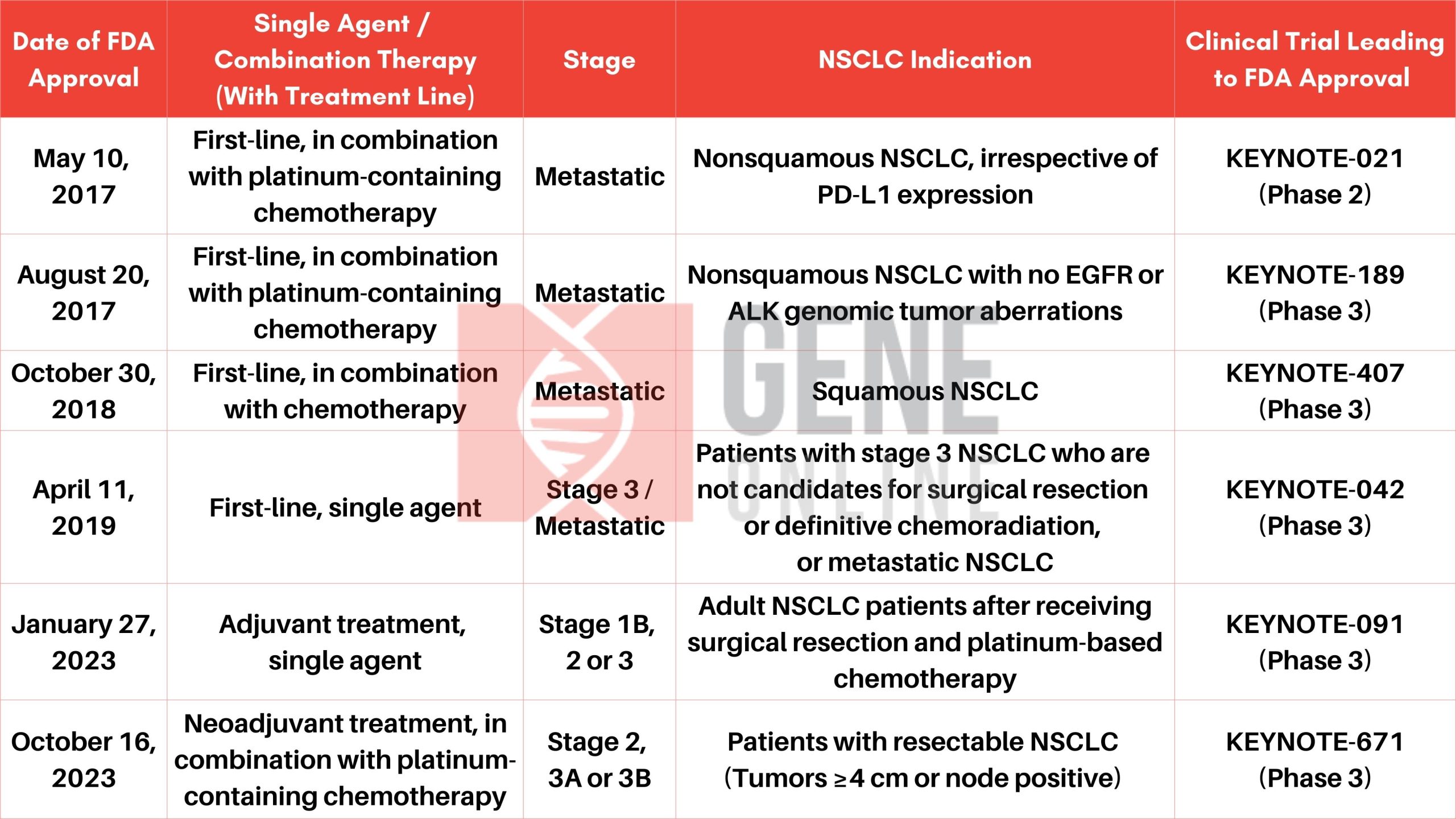
Merck’s KEYTRUDA Receives the Sixth FDA Approval on NSCLC
Merck, known as MSD outside of the United States and Canada, announced on October 16 that the U.S. FDA has approved KEYTRUDA (Pembrolizumab), Merck’s anti-PD-1 monoclonal antibody medication, for the treatment of patients with resectable (tumors ≥4 cm or node positive) non-small cell lung cancer (NSCLC) in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery. With the latest approval, KEYTRUDA has six indications in NSCLC, across both metastatic and earlier stages of NSCLC (as summarized in the table below).
Thermo Fisher Scientific Acquires Swedish Provider of Next-generation Proteomics Solutions
On October 17, Thermo Fisher Scientific announced its plan to acquire all outstanding common shares and American Depositary Shares of Olink Holding AB, a provider of advanced proteomics discovery and development solutions. As part of the transaction, which is expected to be completed by mid-2024, Thermo Fisher said it would pay Olink shareholders $26 per share, while Olink will receive approximately $3.1 billion, which includes approximately $143 million in net cash. With operations in the Americas, Europe and Asia Pacific, the Swedish company provides high-throughput protein analysis using its proprietary technology called Proximity Extension Assay (PEA).
UCB Secures Two FDA Approvals in a Row in a Single Week
Over the past week, UCB secured two consecutive FDA approvals for their new medications based on their favorable Phase 3 clinical trial readouts. On October 17, the Brussels-based company announced that ZILBRYSQ (zilucoplan), a subcutaneous, self-administered macrocyclic peptide complement C5 inhibitor, has been approved by the FDA for treating generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody-positive. On the following day, the Belgian pharma won the FDA approval for BIMZELX (bimekizumab-bkzx), a humanized interleukin-17A and interleukin-17F antagonist for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
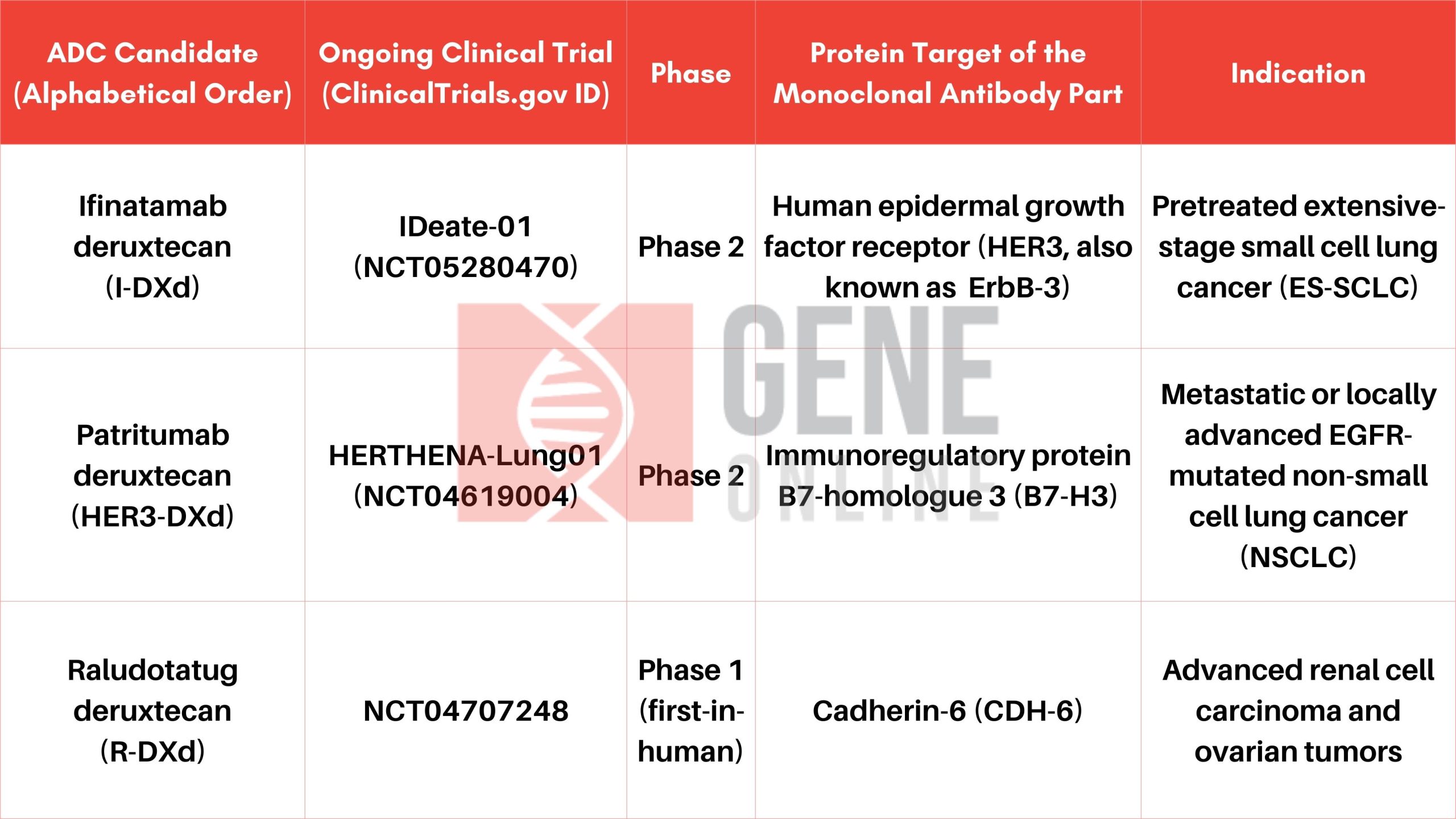
Merck Joins Forces with Daiichi Sankyo in $5.5 Billion Deal to Develop Cancer Drugs
In a strategic move to advance cancer treatment options, pharmaceutical giant Merck (known as MSD outside of the United States and Canada) has agreed to pay $5.5 billion to Daiichi Sankyo for a collaborative effort to jointly develop three promising antibody drug conjugate (ADC) candidates for the treatment of solid tumors. This deal could potentially be worth up to $22 billion to Daiichi Sankyo, depending on the success of these cell-targeting therapies. These innovative drugs are in various stages of clinical development (as shown in the table below) and offer a multi-billion-dollar commercial revenue potential for both companies by the mid-2030s.