Gilead Sciences Acquires CymaBay for $4.3 billion, Heralding a New Era in PBC Treatment
Gilead Sciences, Inc. announced on February 12 that they have agreed to acquire CymaBay for $4.3 billion. CymaBay’s leading drug candidate, seladelpar, used for the treatment of Primary Biliary Cholangitis (PBC), will strengthen Gilead Sciences’ existing liver drug portfolio.
Primary Biliary Cholangitis, the silent killer of female liver function
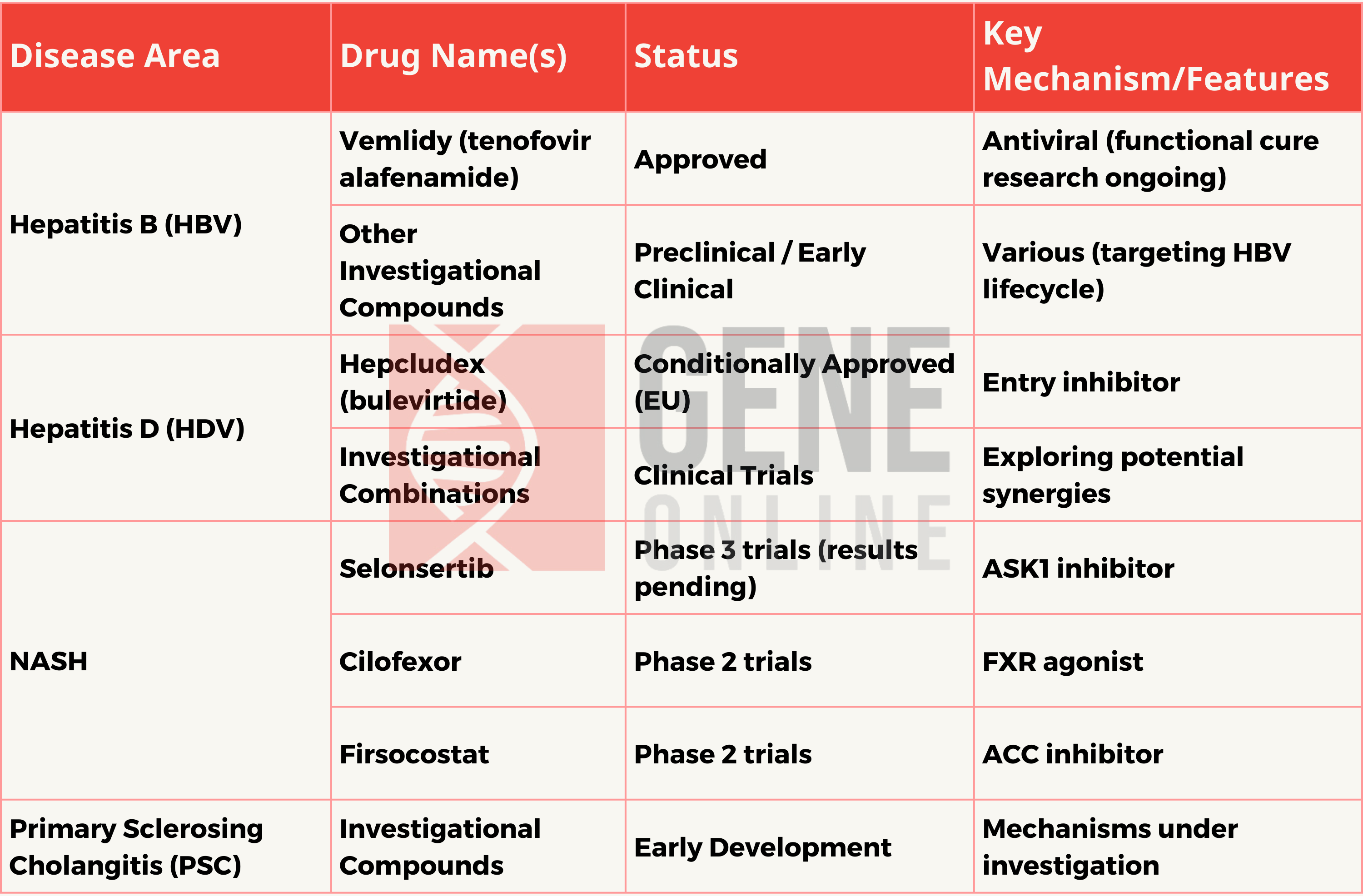
In July 2023, Gilead launched the global ALL4Liver grant program to assist the World Health Organization in achieving its goal of eliminating viral hepatitis by 2030. Gilead’s liver disease medication portfolio includes treatments for Hepatitis B (HBV), Hepatitis D (HDV), Metabolic Dysfunction Associated Fatty Liver Disease (MASH or NASH), and Primary Sclerosing Cholangitis, demonstrating the company’s ongoing commitment to chronic liver diseases.
PBC is a rare “chronic cholestatic liver disease” that gradually damages the bile ducts in the liver of women (1 in every 1,000 women over the age of 40), impairing normal bile flow. As bile accumulates, it leads to inflammation and fibrosis, with common early symptoms being itching and fatigue, eventually progressing to cirrhosis, liver failure, and possibly requiring a liver transplant.
The etiology of PBC is not fully understood but is thought to involve genetic factors and environmental triggers leading to an autoimmune attack on bile duct cells. It mainly affects middle-aged women but can also occur in men. Symptoms vary widely and may include fatigue, itching, dry eyes and mouth (Sjögren’s syndrome), upper right abdominal discomfort, and skin darkening, with more severe complications like jaundice, edema, ascites, and osteoporosis developing as the disease progresses.
Phase 3 RESPONSE Trial Shows Significant Efficacy in PBC
Seladelpar is an orally administered selective peroxisome proliferator-activated receptor delta (PPARδ) agonist. PPARδ, a transcription factor, primarily functions in fat burning and anti-inflammatory effects in the human body, effectively inhibiting the production of inflammatory chemicals for PBC disease treatment.
In the pivotal Phase 3 RESPONSE trial, seladelpar achieved statistical significance in primary composite endpoints of biochemical response (61.7% for the seladelpar trial group vs. 20.0% for placebo), normalization of alkaline phosphatase at 12 months (25.0% for the seladelpar trial group vs. 0.0% for placebo). For patients with moderate to severe itching sustained for 12 months, there was significant improvement in itching symptoms after 6 months.
Seladelpar Receives Positive Review from FDA and EMA
The United States Food and Drug Administration (FDA) has completed its review and accepted the New Drug Application (NDA) for seladelpar, granting Priority Review status, with the Prescription Drug User Fee Act (PDUFA) date set for August 14 this year (2024). Seladelpar’s designation as a Breakthrough Therapy by the FDA indicates significant innovation and potential clinical advantages for PBC patients without cirrhosis or in the compensated cirrhosis stage.
In Europe, the European Medicines Agency (EMA) has granted seladelpar PRIME status to facilitate the drug’s development and review process, providing PBC patients with faster access. Additionally, seladelpar has received Orphan Drug designation in both the United States and Europe.
Daniel O’Day, Chairman and CEO of Gilead Sciences, stated, “We look forward to advancing the development of seladelpar with Gilead Sciences’ knowledge and experience in treating liver diseases. Based on the good research and development results from the CymaBay team so far, we have the potential to address significant unmet needs for PBC patients.”









