GSK Completed the Acquisition of Aiolos Bio, Enhancing the Respiratory Portfolio of Asthma
Following the GlaxoSmithKline (GSK) announcement to buy asthma drugmaker Aiolos Bio for $1.4 billion at the J.P. Morgan Healthcare Conference, GSK completed the acquisition of Aiolos Bio on 15 February 2024.
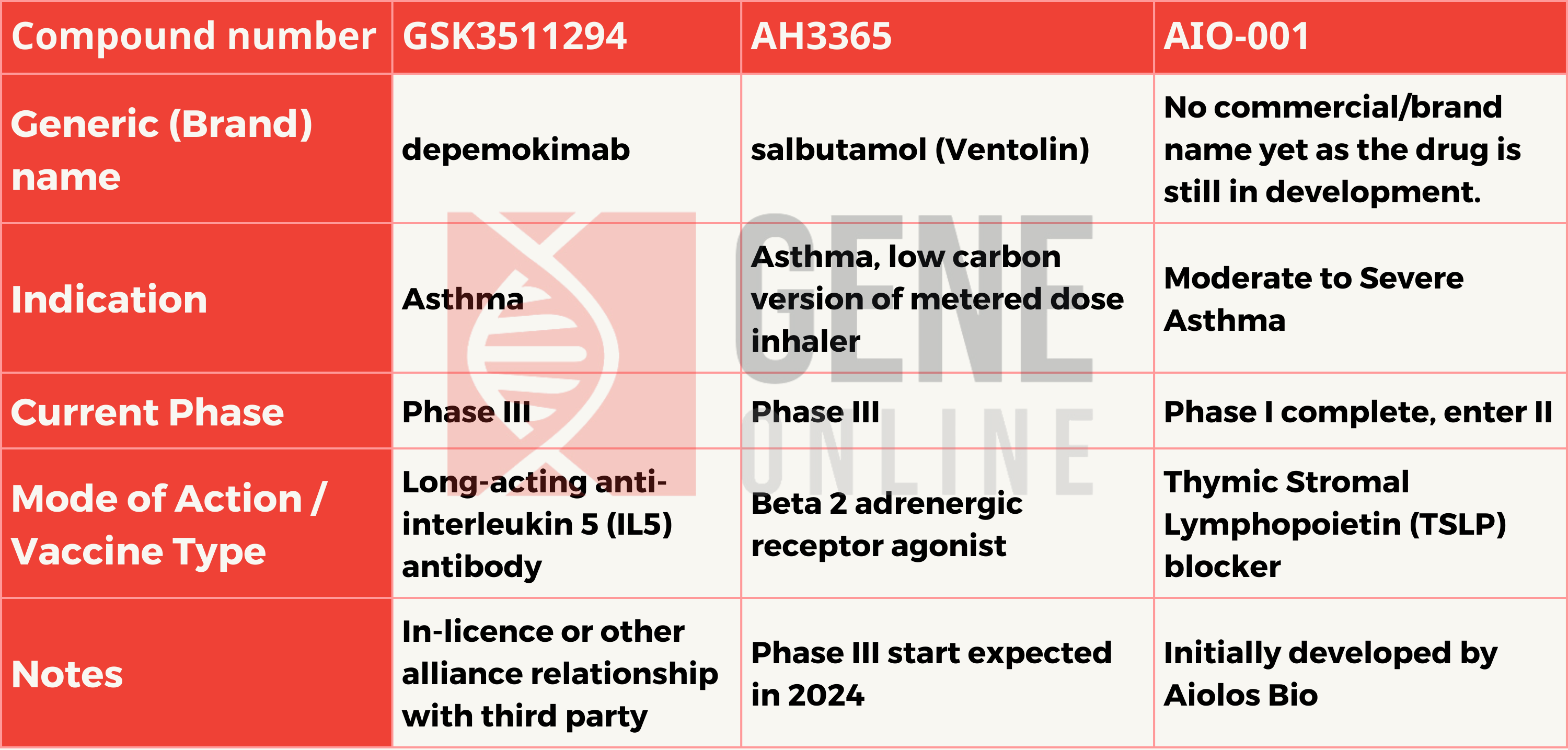
The acquisition includes AIO-001, a potentially best-in-class, long-acting anti-thymic stromal lymphopoietin (TSLP) monoclonal antibody for treating asthma. The terms of agreement includes a $1 billion upfront payment and up to $400 million in certain success-based regulatory milestone payments. Through this acquisition, GSK’s respiratory portfolio expands and complements its existing medicines and vaccines for respiratory conditions.
A Closer Look at AIO-001: A Novel Antibody Targeting Asthma Inflammation
At the heart of AIO-001’s mechanism of action lies a protein called Thymic Stromal Lymphopoietin (TSLP). TSLP is a cytokine, a type of signaling molecule in the immune system, responsible for igniting a cascade of inflammatory events. It plays a particularly important role in allergic inflammation, a hallmark of asthma.
As a monoclonal antibody, AIO-001 is a laboratory-produced molecule that functions like naturally-occurring antibodies in the immune system. AIO-001 is engineered to specifically recognize and bind with high affinity to TSLP. This binding neutralizes TSLP, preventing it from activating its receptor on immune cells.
The potential benefits of AIO-001’s upstream intervention are significant. By targeting TSLP, AIO-001 may reduce the production of a wide range of inflammatory cytokines, dampen the recruitment of immune cells involved in allergic inflammation, and lessen the airway changes often associated with chronic asthma. Further, thanks to its unique ability to neutralize TSLP over extended periods, AIO-001 could offer very infrequent dosing schedules, a potential improvement for patient convenience.
While these prospects are exciting, it’s essential to remember that AIO-001 is still an investigational drug. Ongoing clinical trials are necessary to determine its long-term safety and efficacy thoroughly. Yet, AIO-001 represents a potentially groundbreaking approach to asthma treatment, highlighting the exciting advancements in targeting specific immune pathways that drive this complex disease.
AIO-001 now is exclusively licensed to Aiolos for development and commercialization rights outside of Greater China by Jiangsu Hengrui Pharmaceuticals Co., Ltd. (Hengrui).









