New Technology and Nomenclature to Lead the Transformation of Fatty Liver Disease Diagnosis
Non-alcoholic fatty liver disease (NAFLD) is the world’s most common chronic liver disease, with studies estimating that 32% of adults worldwide are living with this increasingly prevalent condition. When excess fat persists in liver tissues, it can lead to chronic inflammation over time, which is also known as non-alcoholic steatohepatitis (NASH). If left unchecked, the condition may progress to fibrosis or cirrhosis, and eventually to fatal hepatocellular carcinoma or liver failure.
Currently, liver biopsy is the only way to accurately diagnose NASH. However, due to the invasive nature of the procedure and the risk of serious complications, many people feel hesitant to undergo biopsies, complicating physicians’ diagnostic efforts. Nonetheless, according to a recent article published in the medical journal Liver Research, a group of Chinese scientists is developing a diagnostic solution based on sequential ultrasound molecular imaging (USMI). The team has successfully used this technique to identify different grades of hepatic steatosis and inflammation in mice, which, if ultimately applied to humans, could pioneer a new era of diagnosing NASH accurately and non-invasively.
Related article: Breakthrough Stem Cell Treatment Shows Promise in Reversing Liver Disease
Non-Invasive Methods Face Limitations, Leaving Only Biopsy for Accurate Diagnosis of NASH
Currently, fatty liver can usually be detected by abdominal ultrasound, and physicians can also perform liver function tests to determine whether inflammation occurs. However, in order to accurately diagnose NASH, physicians need to rule out viral hepatitis (e.g., hepatitis B or C), alcoholic hepatitis, or autoimmune disorders, as well as confirm that the patient is experiencing symptoms such as lobular inflammation and ballooning degeneration (a phenomenon caused by the gradual destruction of liver cells due to the accumulation of fat). Unfortunately, these symptoms cannot be diagnosed by non-invasive (minimally invasive) methods alone. As a result, a liver biopsy remains the gold standard of NASH diagnosis.
Moreover, even though hepatitis has been detected in a patient with fatty liver, a biopsy is currently the only way to ascertain whether the condition has deteriorated to hepatic fibrosis. Patients’ acceptance of biopsy is generally low due to its invasive nature. Besides, given the fact that only about 1/50000 of the whole liver tissue is sampled during a liver biopsy, there is the possibility of sampling bias (i.e., misdiagnosis because the sampling site happens to be unaffected by steatosis). Therefore, the development of non-invasive modalities for the diagnosis of NASH and liver fibrosis has been a goal of research by scientists worldwide in recent years.
Non-invasive Grading of Hepatic Steatosis and Inflammation by Sequential USMI
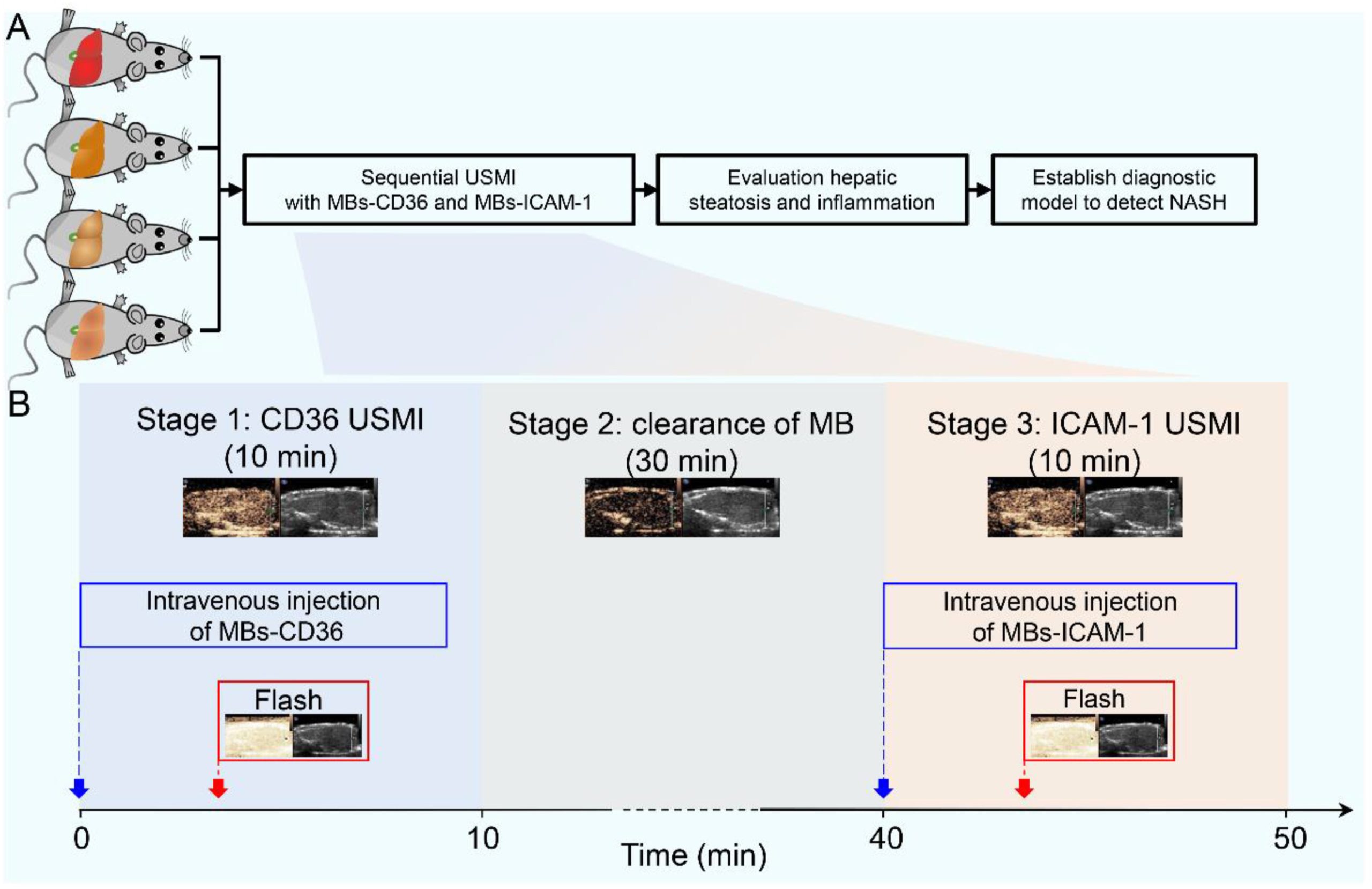
The above-mentioned Chinese research team consisted of scientists from the University of Electronic Science and Technology of China, Sun Yat-sen University, and Southern Medical University. The above-mentioned Chinese research team consisted of scientists from the University of Electronic Science and Technology of China, Sun Yat-sen University, and Southern Medical University. For the diagnosis of NASH by sequential USMI, the team first prepared two types of microbubbles as ultrasound contrast agents. Apart from enhancing the intensity of ultrasound reflection for better image contrast and resolution, the two types of microbubbles also separately target intercellular adhesion molecule-1 (ICAM-1) and CD36 proteins in liver cells. CD36, as a fatty acid translocase, is closely associated with liver steatosis, while ICAM-1 levels increase with the progression of liver inflammation.
Next, the researchers performed the animal study in three stages. First, the mice were injected with microbubbles targeting CD36 ( MBs-CD36) and subjected to the first USMI. After waiting 30 minutes for the elimination of MBs-CD36, the mice were injected with ICAM-targeted microbubbles (MBs-ICAM-1) and the second round of USMI was executed. The ultrasound images obtained were then analyzed in-depth to assess the severity of hepatic steatosis and inflammation in the mice.
The results of the study showed that the USMI approach could recognize different grades of hepatic steatosis and inflammation with a sensitivity and specificity of 95% and 97%, respectively. Tinghui Yin, senior author of the article, indicated that this novel technique has the potential to evolve into a non-invasive diagnostic tool for NASH, which will not only assist physicians in making accurate clinical treatment decisions, but may also be useful in drug discovery and development for NASH.
From N to M: New Nomenclature Transforms Fatty Liver Disease Care
Beyond the above-referenced developments in non-invasive diagnostic techniques, another major update related to the diagnosis and treatment of NAFLD in recent years has been the renaming of the disease. The origin dates back to 2019 when scientists proposed changing the name of NAFLD to MAFLD (metabolic dysfunction-associated fatty liver disease).
While it appears to be a change of a single alphabet in abbreviation, the underlying meaning is quite different. First, removing the word “alcoholic” helps to eliminate the stigma associated with alcohol consumption and its effects on the liver and reduces the number of cases where patients’ unwillingness to disclose their true level of alcohol consumption interferes with physicians’ judgment during the consultation. Also, since alcohol consumption can occur in many different contexts (e.g., some people drink only occasionally), medical professionals have expressed concern that it may not be objective to categorize fatty liver disease according to whether a patient has been drinking excessively over a long period of time. Furthermore, not mentioning “non-alcoholic” would prevent patients from wrongly misperceiving the disease, thereby trivializing its severity.
On the other hand, with the change in nomenclature, the diagnostic focus has shifted to patients’ metabolic conditions. A patient with liver steatosis and one of the metabolic abnormalities such as overweight (BMI 23 or above), hypertension, hyperlipidemia, and type 2 diabetes will meet the new definition of diagnosis. Medical professionals believe that such an update will raise public awareness of the dangers of MAFLD, encouraging more people to seek medical attention as soon as they notice abnormalities in their metabolic indexes, which will be of great benefit to disease prevention and treatment.
After much deliberation by international experts, the renaming proposal was finally passed in 2020. Based on the same logic, NASH was also renamed as MASH (metabolic dysfunction associated-steatohepatitis). With the advancement of diagnostic criteria, diagnostic tools, clinical research and treatments, there is hope that different groups of fatty liver disease will be adequately addressed to reduce the public health threat posed by MAFLD or MASH.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com










