Resmetirom Offers Hope for MASH Patients: Positive Phase 3 Results for Liver Fibrosis
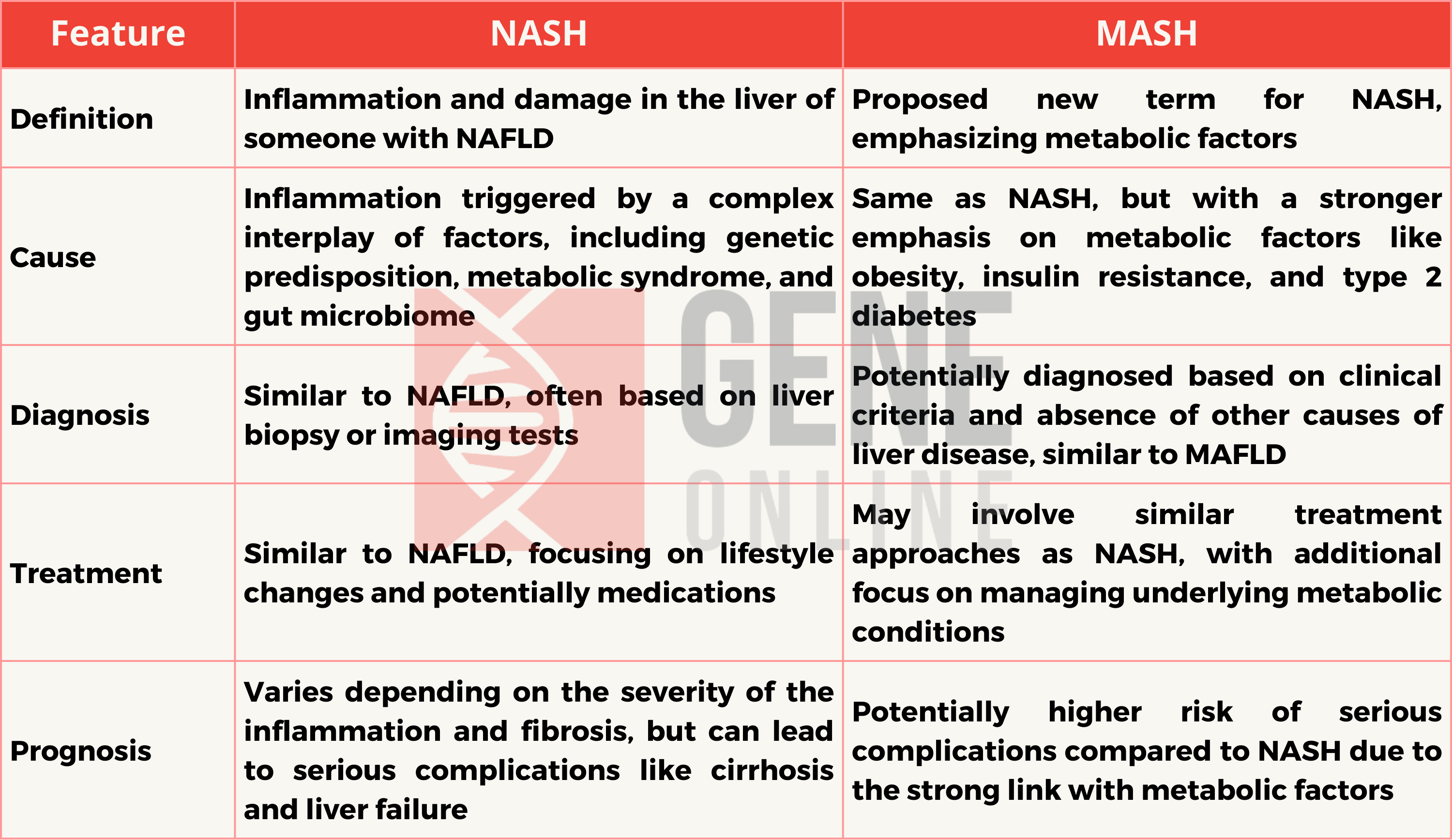
Nonalcoholic steatohepatitis (NASH) , now renamed as metabolic dysfunction associated-steatohepatitis (MASH), is a silent but progressive liver disease, often an advanced form of Nonalcoholic fatty liver disease (NAFLD) or Metabolic-dysfunction associated fatty liver disease (MAFLD).
Characterized by inflammation and damage resulting in liver fibrosis, MASH can advance to cirrhosis or liver cancer, presenting a significant healthcare challenge due to its stealthy progression and lack of approved pharmacological treatments. However, a new beacon of hope has emerged with the unprecedented success of the phase 3 trial of resmetirom, a selective thyroid hormone receptor beta agonist.
Key Differences of NASH and MASH
MASH: One of the Main Cause of Liver Fibrosis
The prevalence of MASH has been rising globally, mostly in parallel with the obesity and diabetes pandemics. It primarily affects individuals with a spectrum of metabolic syndromes such as insulin resistance, dyslipidemia, and hypertension. The lack of symptoms in the early stages makes it hard to diagnose, often coming into the spotlight only when significant liver damage has occurred.
Liver fibrosis, a hallmark consequence of MASH, signifies the accumulation of scar tissue as a result of persistent inflammation, eventually leading to the disruption of the liver’s architecture and function. With poor outcomes such as cirrhosis, liver failure, and hepatocellular carcinoma on the horizon, the urgency for a therapeutic intervention is undeniable.
Resmetirom as a Potential Treatment for MASH: Phase 3 Trial Results
The phase 3 trial of resmetirom, a novel therapeutic agent targeting MASH with liver fibrosis which can improve fat metabolism and mitochondrial function but also reduced lipotoxicity and inflammation, yielded promising results over a 52-week period. The trial included almost a thousand adults with biopsy-confirmed MASH, who were administered daily doses of 80 mg or 100 mg of resmetirom or a placebo for comparison. Resmetirom demonstrated significant effectiveness in resolving MASH without worsening fibrosis, with resolution rates of 25.9% for the 80 mg group and 29.9% for the 100 mg group, far exceeding the 9.7% resolution rate in the placebo group.
Improvement in fibrosis by at least one stage without a worsening NAFLD activity score was also significant, with 24.2% in the 80 mg group and 25.9% in the 100 mg group, compared to 14.2% in the placebo group. Furthermore, participants saw a substantial drop in LDL cholesterol levels—another positive side effect considering the dyslipidemia often occurring alongside MASH. Despite some gastrointestinal side effects, such as diarrhea and nausea, serious adverse events were similarly reported across the treatment and placebo groups, suggesting an acceptable safety profile.
The trial results position resmetirom as a potential first-line treatment for MASH with fibrosis, though further long-term studies will be necessary to fully establish its efficacy and safety profile.
New Era in Fighting Against MASH-Related Fibrosis
The trial’s findings pivot resmetirom as a frontrunner in the arena of MASH pharmacotherapy, providing a strong case for its potential role as a first-line treatment in liver fibrosis secondary to MASH. With nearly three decades since the initial conceptualization of MASH as a distinct liver disease entity, the clinical community is on the cusp of a therapeutic breakthrough bolstered by the promising results of the resmetirom Phase 3 trial.
As a concluding remark, though resmetirom’s profile appears encouraging, comprehensive long-term studies and post-marketing surveillance will remain imperative to ensure sustained effectiveness and safety. Similarly, as with all trials, the translatability to real-world populations will be a crucial determinant of resmetirom’s ultimate place in clinical practice.
Nevertheless, the tide is turning in the war against MASH and related fibrosis, with resmetirom etching its name as a potential cornerstone in the management of this pervasive and pernicious disease.
Note: NASH and MASH, NAFLD and MAFLD are both now interchangeable.











