Scientists Study Imaging Probes in First-Ever Amputated Human Limb Model
Recently, researchers from the United States developed a novel, advanced system to identify the fluorescent agents with the highest chance of clinical success. Their study aims to improve the accuracy and safety of fluorescent agents and, more importantly, minimize potential harm to patients.
The finding is reported in the Journal of Biomedical Optics (JBO) and is the first-in-kind model to be used for lead fluorescent agent selection in the future.
Related Article: Targeting Metabolism for Noninvasive Multi-Cancer Early Detection Tests
The Next Era of Fluorescence-Guided Surgery
Fluorescence-guided surgery (FGS) is an imaging technique that allows the surgeon to visualize different structures and types of tissue during a surgical procedure that may not be as visible under white light conditions.
The field is growing rapidly due to its various advantages compared to traditional clinical imaging techniques, such as its higher contrast and sensitivity, less subjective use, and ease of instrument operation. The research interest in FGS continues to grow over various key aspects, including fluorescent probe development, surgical system development, as well as its potential clinical applications.
In FGS, tissues of interest are targeted and labeled using special molecules called fluorophores. The fluorophores aim to distinguish the target tissue from other tissues and guide surgical steps. There are only three FDA-approved fluorophores for clinical use so far, including indocyanine green (ICG), fluorescein, and methylene blue (MB). However, the main challenge is they are untargeted, which limits their specificity.
Hence, identifying new, specific fluorophores that target certain tissues is of the utmost need. Although many candidate fluorophores showed satisfying results in animal models, implementing data into real human models is still required.
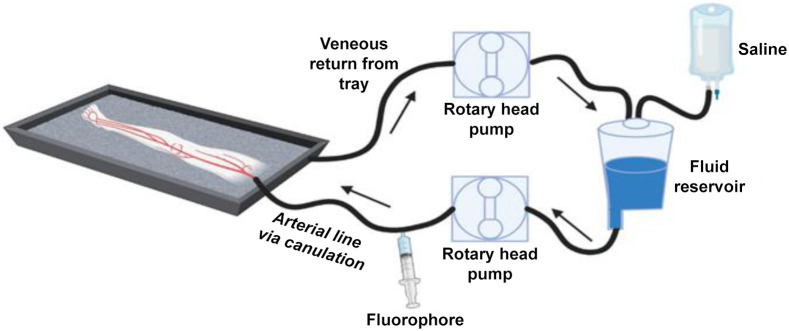
Human Limb Model to Study Fluorophore Specificity
In this study, the researchers developed an amputated human lower limb model for testing a nerve-specific fluorophore. According to their design, tissue examination is started soon after amputation, before the tissue breakdown happens. In addition, the team mimicked the vascular and osmotic pressure seen in live human tissues by using a cardiac pump to perfuse saline.
After amputation, the limbs are transported to a surgical laboratory to be perfused with the targeted fluorophore using a recirculated loop. First, saline is perfused through a dominant artery, and then a standard dose of the nerve-specific LGW 16-03 fluorophore is administered. The limb is gravity-drained, and the collected perfusate is recycled back into the circuit to mimic blood circulation.
The nerve tissue is imaged in situ and ex vivo for half an hour. As a result, the fluorophore showed excellent signal-to-background ratio (SBR) performance, which indicates the strength of the desired signal relative to the background noise.
To sum up, the study introduced the first human model used to study and select fluorescent agents and is believed to have the potential to study peripheral diseases and pathological features in tissues under controlled conditions. Moreover, the scientists also plan to apply the platform in cancer research by studying the changes caused by tumor growth.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com










