The Emergence of Metabolic Therapy, Unlocking a Next-Generation Cancer Treatment Strategy
Cancer cells acquire exceptional survivability through genetic mutations and altered molecular signaling, enabling them to grow faster, cause damage to normal tissues, induce angiogenesis for blood supply, and escape from cell clearance processes such as apoptosis and immunity. Currently, most of the targeted cancer drugs are based on blocking abnormal signaling pathways as a means of suppressing cancer growth, for example, Iressa, Crizotinib, and Avastin (Bevacizumab).
Apart from blocking abnormal signaling pathways in cancer cells, new drugs targeting cancer metabolism have aroused attention in recent years, and their theoretical basis is derived from the renowned Warburg effect in the field of cancer research. Nearly a century ago, Nobel Laureate Otto Heinrich Warburg discovered that cancer cells produce energy in a very different way than normal cells, utilizing glycolysis followed by lactic acid fermentation even in the presence of abundant oxygen, and this opened the door to the study of cancer cell metabolism.
Related article: Latest Discovery in Metabolic Pathways of T-Cells Opens New Door to Immunology Research
Ongoing Evolution in Cancer Metabolic Theory and Applications
Cancer cells need energy supply and nutrients on a daily basis to maintain rapid growth and division. Apart from energy, cancer cells must also consume large amounts of cell-building materials such as nucleic acids and amino acids. In this regard, scientists have developed various methods to label and kill tumor cells.
Nowadays, various studies on metabolic processes of cancer cells and related diagnostic and therapeutic applications in the field of cancer treatment are already quite mature and widespread, such as the use of F-18 fluoro-2-deoxyglucose positron emission tomography (FDG-PET) for cancer screening, or the use of fluorouracil (5-FU), which induces DNA damage by depleting nucleotide material essential for DNA replication and repair, as a first-line drug for many types of metastatic cancers. These diagnostics and therapies have been developed based on the theory of metabolic processes of cancer.
Inspired by the concept of tumor microenvironment (TME), cancer metabolic research is no longer limited to the internal processes that occur in cancer cells, but also includes oxygen content, metal ions, nutrient molecules, vascular epithelium, blood supply, fibroblasts, and microorganisms in the surrounding tissues, all of which affect the growth strategies of cancer cells.
The TME theory also suggests that the microenvironment can shape the metabolic and molecular signals of tumors, and the complex physiological regulatory mechanisms have led to a shift in the development of anticancer drugs from monotherapy to combination therapy of multiple drugs. Therefore, although there are a few trials of single-agent metabolic inhibitors in clinical research, most of them use conventional chemotherapeutic drugs in combination with targeted metabolic therapies to enhance the effect on cancer cell destruction.
Recent breakthroughs in cancer treatment by invigorating the immune system have led to an emphasis on research in cancer immunology, and James P. Allison and Tasuku Honjo, who discovered important cancer immune switches, were awarded the Nobel Prize in Physiology or Medicine for their work. Nutrient molecules in the tumor microenvironment are also known to be involved in regulating the role of immune cells, and therefore many clinical trials of cancer metabolism drugs have combined therapeutic strategies with immune checkpoint inhibitors in the hope of destroying cancer cells by both internal and external attack.
The American Association for Cancer Research (AACR) Annual Meeting 2023 concluded in April with one of the major symposia on “Immunometabolism in the Tumor Microenvironment”. Given that metabolic effects have broad implications, research related to cancer metabolism can also be found in various sub-symposia, such as signaling pathways, diagnosis and prognosis, radiation therapy, and cancer evolution.
The Only Taiwanese Company with a Long-Term Commitment to Clinical Cancer Metabolic Therapy
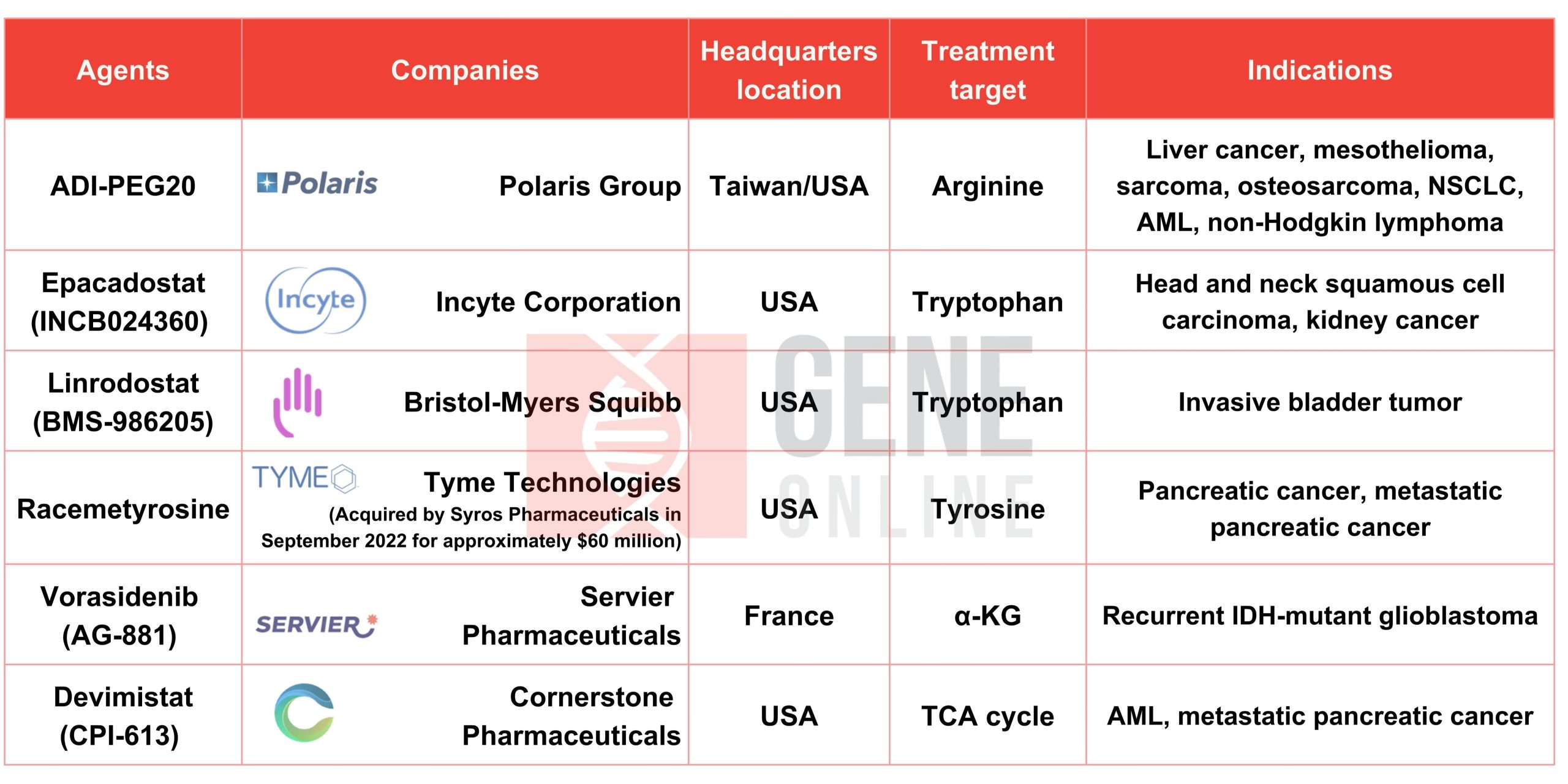
Among drugs targeting cancer metabolism that have entered clinical studies, ADI-PEG20 (Pegargiminase) is the only drug developed by a Taiwanese biotech company, Polaris Pharmaceuticals, and is the world’s first synergistic therapy that combines targeted arginine metabolism with platinum-based chemotherapeutics.
Pegargiminase is a PEGylated therapeutic protein based on a microbial arginine catabolizing enzyme called arginine deiminase (ADI) with polymer chains of polyethylene glycol attached to it. The drug exploits the lack of self-synthesis of arginine in specific cancer cells to break down and deplete circulating arginine in patients, thereby inducing cell cycle arrest, apoptosis or autophagy in cancer cells. Normal cells, however, can remain unaffected by this treatment because they can synthesize arginine on their own.
In animal studies, ADI-PEG20 not only increased the sensitivity of brain cancer cells to radiation, but also induced macrophages to penetrate into brain tumor structures and fight against tumor cells.
According to a report presented at the AACR’s Clinical Trials Plenary Session meeting, 249 patients with chemonaïve invasive non-epithelioid pleural malignant mesothelioma were enrolled in the phase 2/3 clinical study called ATOMIC-Meso. Patients were assigned to the ADI-PEG20 and placebo groups, while also receiving folate antimetabolites (Pemetrexed) and platinum-based chemotherapy drug (Cisplatin).
The results showed that ADI-PEG20 significantly prolonged median survival, with 9.3 and 7.7 months (95% confidence interval [CI], 7.9-11.8/6.1-9.5) in the ADI-PEG20 and placebo groups, respectively, and median progression-free survival, with 6.2 and 5.9 months (95% CI, 5.8-7.4) in the ADI-PEG20 and placebo groups, respectively. The ADI-PEG20 group had a 29% lower risk for death (hazard ratio [HR] for death, 0.71; p = 0.023) and a 35% lower risk for disease progression. In addition, a 1-year overall survival analysis showed that treatment with ADI-PEG20 yielded 10% more than placebo (41.4 vs. 31.4%).
In terms of safety, ADI-PEG20 was well tolerated, with a similar proportion of patients experiencing treatment interruptions due to side effects in both groups, 20.7% in the ADI-PEG20 group and 13.7% in the placebo group, and no new safety issues were identified compared to previous trials.
As disclosed in the clinicaltrials.gov, the clinical trial registry in the U.S., ADI-PEG20 is being tested in trials targeting various indications such as acute myeloid leukemia, liver cancer, non-small cell lung cancer, glioblastoma, and soft tissue sarcoma, with trial designs ranging from targeting specific genetic groups to immune checkpoint drugs or combination with radiation therapy. In the future, it is anticipated that the cancer groups suitable for arginine deprivation therapy will be identified.
Progress of Phase 2 and 3 Global Clinical Trials of ADI-PEG20
Two clinical phase 3 trials involving ADI-PEG20 are currently underway for liver cancer and soft tissue sarcoma in the United States and Taiwan. The liver cancer trial in Taiwan enrolled the first patient at Linkou Chang Gung Memorial Hospital last June and is expected to be unblinded in 2024. The Phase 2 trial of soft tissue sarcoma, which includes the University of Washington, Stanford University, Columbia University Medical School, and the University of Southern California Cancer Center, has received a grant from the National Institutes of Health, and is currently enrolling patients in the Phase 3 trial to further target Leiomyosarcoma, an invasive or metastatic form of soft tissue sarcoma. The study is scheduled to start this June.
Currently FDA-approved Drugs for Cancer Metabolic Therapy
The FDA granted approval to enasidenib and ivosidenib, inhibitors of isocitrate dehydrogenase (IDH), a key enzyme of the tricarboxylic acid cycle (TCA cycle) associated with energy metabolism, in 2017, 2019 and 2021 for the treatment of patients with acute myeloid leukemia (AML) with IDH1 mutations and cholangiocarcinoma.
Among drugs under investigation in clinical studies of different phases regarding cancer metabolism, the targeted metabolic pathways include those involving glucose, lactate, amino acids (glutamine, aspartate, arginine, tyrosine and tryptophan), and fatty acids.
Several high-profile clinical trials of breakthrough drugs of cancer metabolic therapy were announced at this year’s AACR Annual Meeting, while Polaris’ ADI-PEG20 was one of them. Other notable new drugs include Lilly Oncology’ LY3410738 (IDH 1/2 inhibitor), Dracen Pharmaceuticals’ DRP-104 (glutamine antagonist), and Cornerstone Pharmaceuticals’ CPI-613 (mitochondrial metabolism inhibitor targeting TCA cycle).
Original article written by Justine Zhu, translated by Richard Chau.
©www.geneonline.com All rights reserved. Collaborate with us: service@geneonlineasia.com










